2024
Original
Paper
1. Selective Recognition
between Aromatics and Aliphatics by Cage-Shaped
Borates Supported by Machine Learning Approach
Tsutsui, Y.; Yanaka, I.; Takeda, K.; Kondo,
M.; Takizawa, S.; Kojima, R.; Konishi, A.; Yasuda, M. Org. Biomol.
Chem. 2024, in press (DOI).
2. In-Silico-Assisted
Derivatization of Triarylboranes for the Catalytic
Reductive Functionalization of Aniline-Derived Amino Acids and Peptides with H2
Hisata, Y.; Washio, T.; Takizawa, S.;
Ogoshi, S.; Hoshimoto, Y. Nat. Commun. 2024, in press.
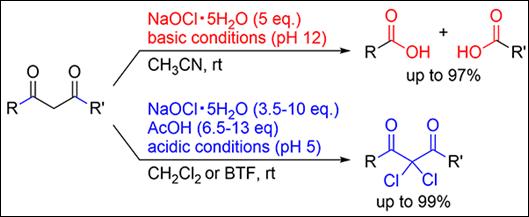
3. Product Selectivity
Control under Acidic and Basic Conditions on Oxidative Transformation of
1,3-Dicarbonyls Using Sodium Hypochlorite Pentahydrate
Kirihara, M.; Sakamoto, Y.; Tanaka,
T.; Kawai, T.; Okada, T.; Kimura, Y.; Takizawa, S. Synthesis 2024, 56, in press (DOI)
4. Synthesis and Structural
and Optical Behavior of Dehydrohelicene-Containing
Polycyclic Compounds
Khalid, Md. I.; Salem, M. S. H.;
Takizawa, S. Molecules 2024, 29,
296 (DOI).
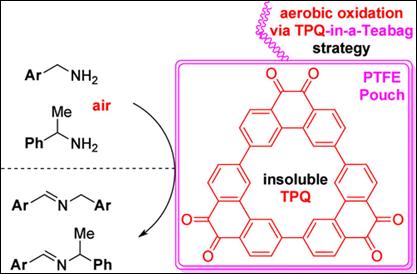
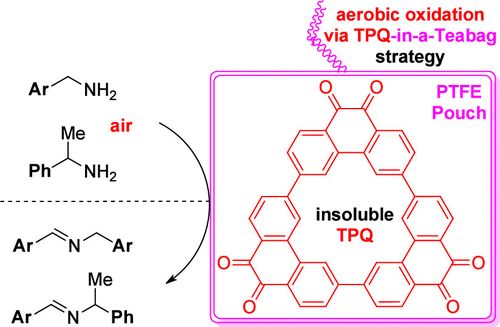
5. Light-induced autoxidation
of aldehydes to peracids and carboxylic acids
Salem, M. S. H.; Dubois, C.;
Takamura, Y.; Kitajima, A.; Kawai, T.; Takizawa, S.; Kirihara, M. Green Chem. 2024, 26, 375 (DOI).
2023
Original
Paper
1.
Electrochemical Carbon-Ferrier Rearrangement Using a Microflow
Reactor and Machine Learning-assisted Exploration of Suitable Conditions
Sato, E.; Tachiwaki,
G.; Fujii, M.; Mitsudo, K.; Washio, T.; Takizawa, S.;
Suga, S. Org. Process Res. Dev.
2023, in press (DOI).
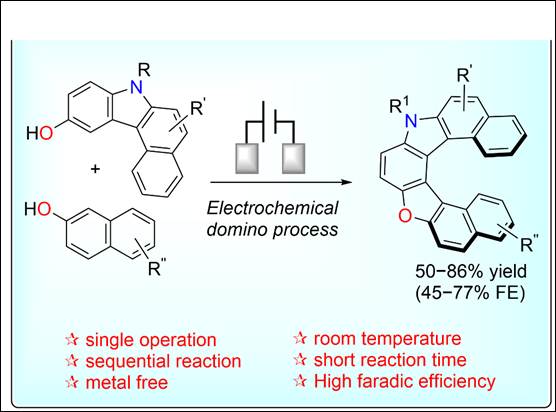
2.
Data-driven Electrochemical One-pot Synthesis of Double
Hetero[7]dehydrohelicene
Salem, M. S. H.; Sharma, R.; Khalid,
Md. I.; Sasi, M.; Amasaki, R.; Imai, Y.; Arisawa, M.;
Takizawa S.
Electrochemistry
2023, 91, 112015 (DOI).
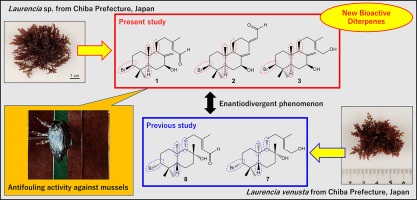
3. Antifouling Brominated Diterpenoids from Japanese Marine Red Alga
Laurencia venusta Yamada
Fukada, R.; Yamagishi, Y.; Nagasaka, M.; Osada, D.; Nimura, K.;
Oshima, I.; Tsujimoto, K.; Kirihara, M.; Takizawa, S.; Kikuchi, N.; Ishii, T.; Kamada, T. Chem. Biodiversity 2023, 20,
e202300888 (DOI).
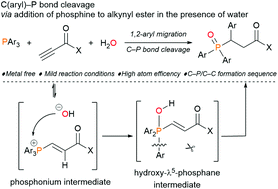
4. Azobenzene─based Chiral Photoswitchable Catalysts
Kondo, M.; Nakamura, K.;
Sasai, H.; Takizawa, S. J. Synth. Org. Chem. Jpn. 2023, 81,
817 (DOI).
5. Light-controlled pKa Value of Chiral
Brønsted Acid Catalysts in Enantioselective Aza-Friedel–Crafts Reaction
Krishnan,
C. G.; Kondo, M.; Yasuda, Y.; Fan, D.; Nakamura, K.; Wakabayashi, Y.; Sasai,
H.; Takizawa, S. Chem. Commun. 2023, 59, 9956 (DOI).
6.
Photoswitchable Chiral Organocatalysts:
Photocontrol of Enantioselective Reactions
Kondo, M.; Nakamura, K.; Krishnan,
C.G.; Sasai, H.; Takizawa, S. Chem. Rec. 2023, 23, e202300040 (DOI).
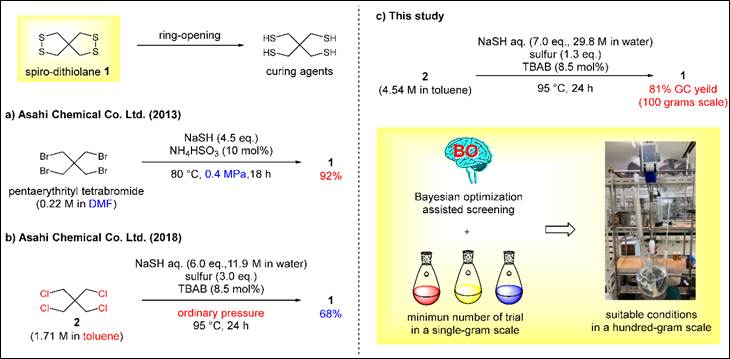
7.
Bayesian Optimization-assisted Screening to Identify Improved
Reaction Conditions for Spiro-dithiolane Synthesis
Kondo, M.; Wathsala, H. D. P.;
Ishikawa, K.; Yamashita, D.; Miyazaki, T.; Ohno, Y.; Sasai, H.; Washio, T.;
Takizawa, S. Molecules, 2023,
28, 5180 (DOI).
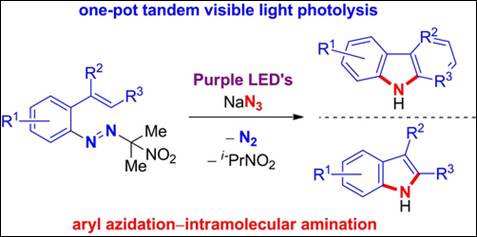
8.
Electrochemical Synthesis of Hetero[7]helicenes Containing
Pyrrole and Furan Rings via an Oxidative Heterocoupling
and Dehydrative Cyclization Sequence
Salem, M. S. H.; Khalid, Md. I.; Sako, M.; Higashida, K.; Lacroix, C.; Kondo, M.; Takishima, R.; Taniguchi, T.; Miura, M.; Vo-Thanh, G.; Sasai, H.;
Takizawa, S. Adv.
Synth. Catal. 2023, 365, 373 (DOI).
9. Two-pot synthesis of
unsymmetrical hetero[7]helicenes with intriguing optical properties
Salem, M. S. H.; Khalid,
Md. I.; Sasai, H.; Takizawa, S. Tetrahedron 2023, 133, 133266 (DOI).

10.
Metal-Free Aerobic C–N Bond Formation of Styrene and Arylamines
via Photoactivated Electron Donor-Acceptor Complexation
Fan, D.; Sabri, A.; Sasai, H.;
Takizawa, S. Molecules 2023, 28, 356 (DOI).
Original
Paper
1.
Bayesian optimization with constraint on passed charge for multiparameter
screening of electrochemical reductive carboxylation in a flow microreactor
Naito, Y.; Kondo, M.; Nakamura, Y.; Shida, N.; Ishikawa, K.;
Washio, T.; Takizawa, S.; Atobe, M. Chem. Commun. 2022, 58, 3893 (DOI) (Selected as Inside Front Cover).
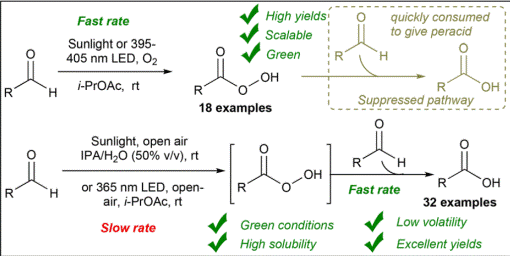
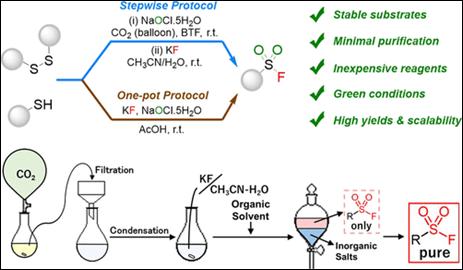
2.
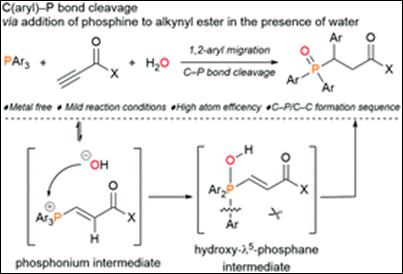
Metal-free C(aryl)–P bond cleavage: experimental and computational
studies of the Michael addition/aryl migration of triarylphosphines
to alkenyl esters
Sako, M.; Kanomata,
K.; Mohamed, S. H. S.; Furukawa, T.; Sasai H.; Takizawa, S. Org. Chem. Front. 2022, 9, 2187 (DOI).

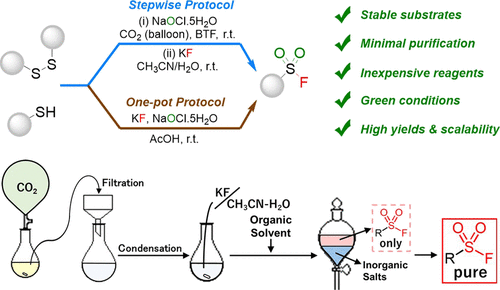
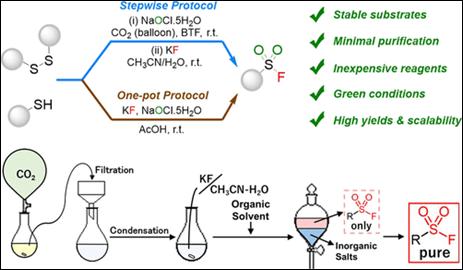
3.
Photoswitchable chiral cation-binding
catalyst: Photocontrol of catalytic activity on enantioselective aminal synthesis
Krishnan, C.; Kondo, M.;
Nakamura, K.; Sasai, H.; Takizawa, S. Org. Lett. 2022, 24, 2670 (DOI) (Selected as Front Cover).
4. DAST-mediated ring-opening of cyclopropyl silyl ethers in nitriles: Facile synthesis of allylic amides via Ritter-type process
Kirihara, M.; Nakamura, R.; Nakakura,
K.; Tujimoto, K.; Mohamed S. H. S.; Suzuki, T.; Takizawa, S. Org. Biomol.
Chem. 2022, 20, 6558 (DOI).
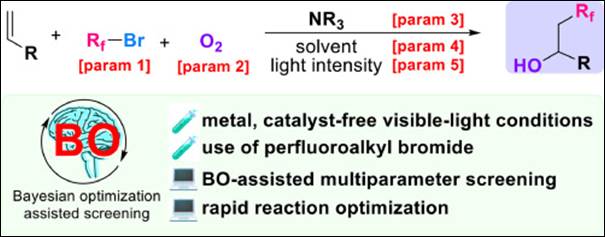
5. New anionic cobalt(III) complexes enable
enantioselective synthesis of spiro-fused oxazoline and iodoacetal derivatives
Salem, M.
S. H. and Takizawa, S. Front.
Chem. 2022, 10:1034291 (DOI).
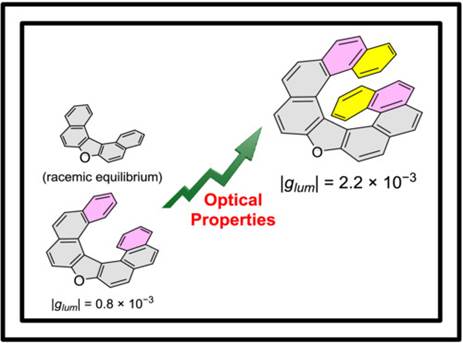
6. Bayesian
optimization-driven parallel-screening on multi-parameters of micromixer-type
and organocatalytic conditions in the flow biaryl
synthesis
Kondo, M.; Wathsala, H. D. P.; Salem, M. S. H.; Ishikawa, H.; Hara, S.; Takaai, T.; Washio, T.; Sasai, H.; Takizawa, S. Commun. Chem. 2022, 5,
148. (DOI)
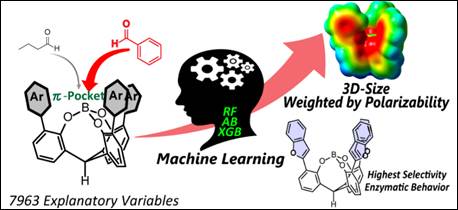
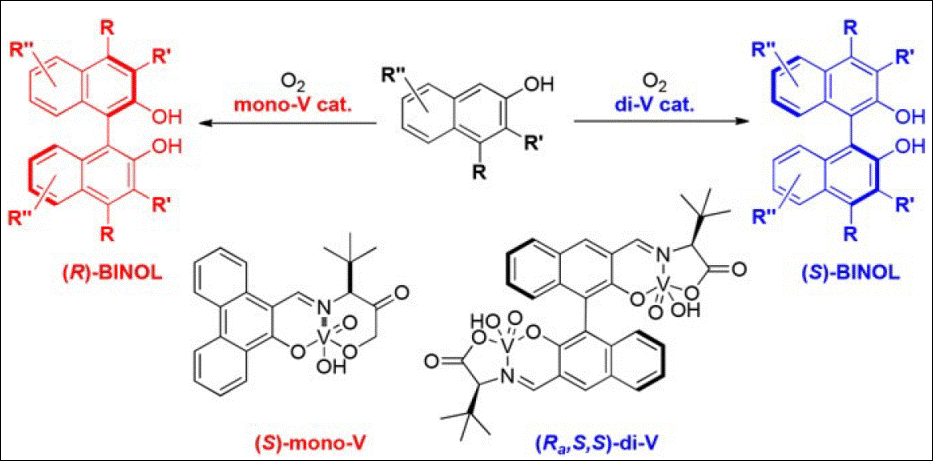
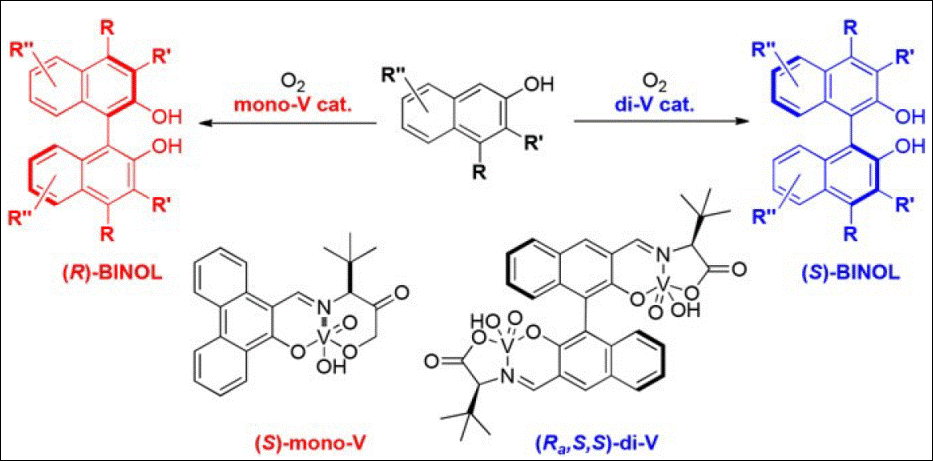
7.
Atroposelective synthesis of C–C
axially chiral compounds via mono- and dinuclear
vanadium catalysis
Kumar,
A.; Sasai, H.; Takizawa, S. Acc. Chem. Res.
2022, 55, 2949 (DOI).

8. Electrochemical synthesis of heterodehydro[7]helicenes
Khalid, Md. I.; Salem, M. S. H.; Sako, M.; Kondo, M.; Sasai, H.; Takizawa, S. Commun. Chem. 2022, 5, 166 (DOI).
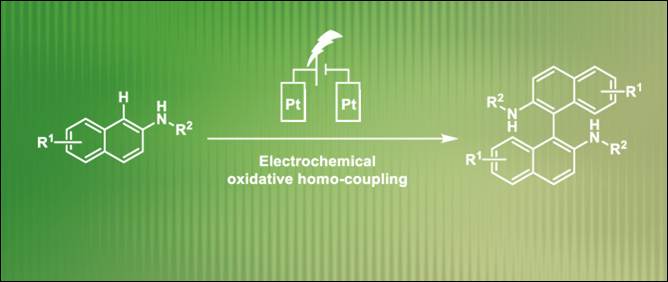
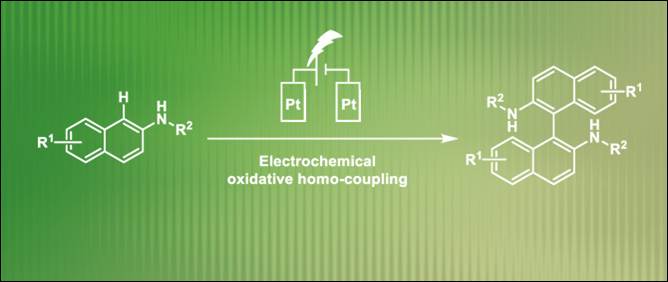
9.
Electrochemical
Synthesis of 1,1’-Binaphthalene-2,2’-diamines via Transition-Metal-Free
Oxidative Homocoupling
Fan, D.; Khalid, Md. I.; Kamble, G. T.; Sasai, H.; Takizawa, S. Sustain. Chem. 2022, 3, 551 (DOI) (Highlighted
on the Main Page of the Journal).
Hiro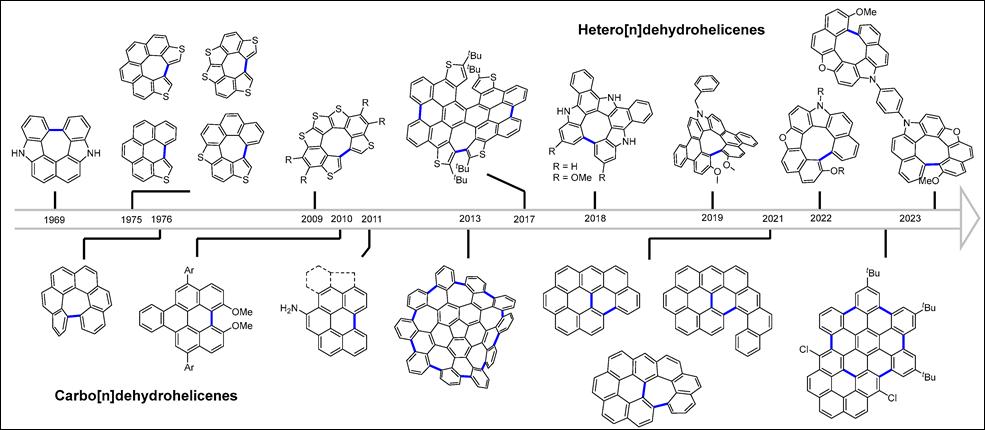
10. Two-Step
Synthesis, Structure, and Optical Features of a Double Hetero[7]helicene
Salem, M.
S. H.; Sabri, A.; Khalid, I.; Sasai, H.; Takizawa, S. Molecules 2022, 27, 9068 (DOI).
2021
Original
Paper
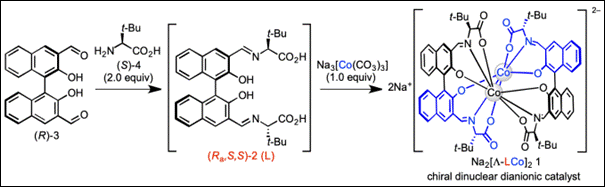
1.
Preparation of Optically Pure Dinuclear Cobalt(III) Complex with Λ–Configuration as a Dianionic Chiral Catalyst
Salem, M. S. H.;
Kumar, A.; Sako, M.; Abe, T.; Takizawa, S.; Sasai, H.
Heterocycles, 2021, 103, 225 (DOI).
2.
Photoswitchable
Chiral Phase Transfer Catalyst
Kondo, M.; Nakamura, K.; Krishnan,
C.; Takizawa, S.; Abe, T.; Sasai, H. ACS
Catalysis, 2021, 11, 1863(DOI).
<Highlighted
in Synfacts 2021, 17, 442. (DOI)>
3.
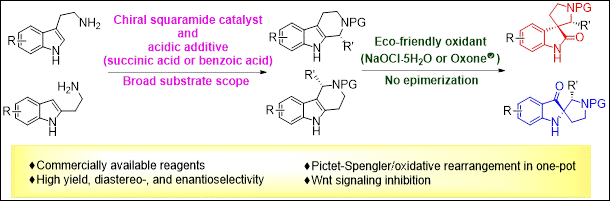
Practical Stereoselective Synthesis of C3‐Spirooxindole‐ and C2‐Spiropseudoindoxyl‐Pyrrolidines via Organocatalyzed Pictet‐Spengler Reaction/Oxidative Rearrangement Sequence
Kondo,
M.; Matsuyama, N.; Aye, T. Z.; Mattan, I.; Sato, T.; Makita, Y.; Ishibashi, M.;
Arai, M.; Takizawa, S.; Sasai, H. Adv. Synth. Catal., 2021, 363, 2648(DOI).
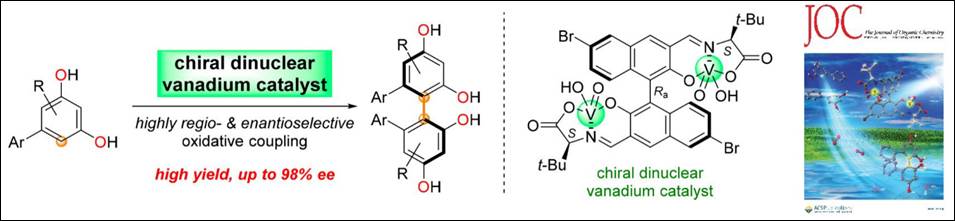
4.
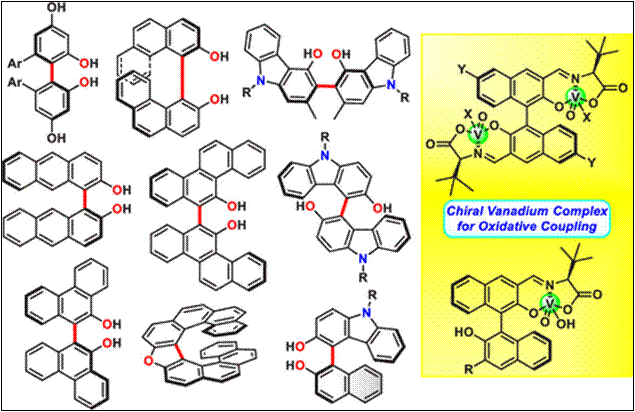
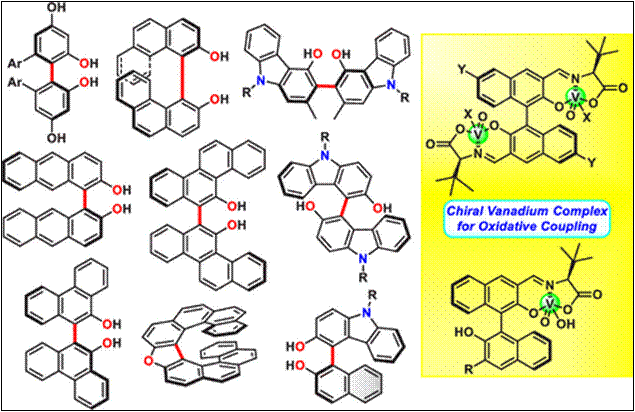
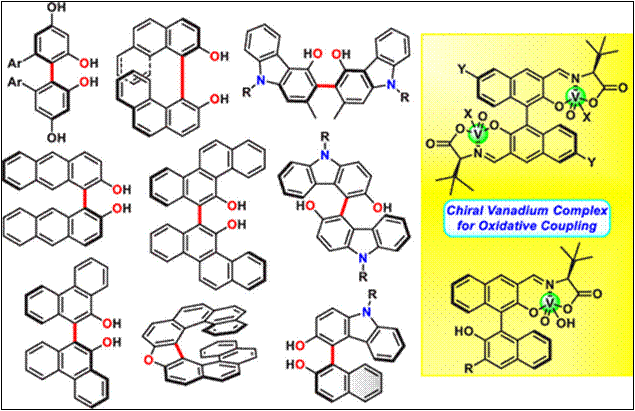
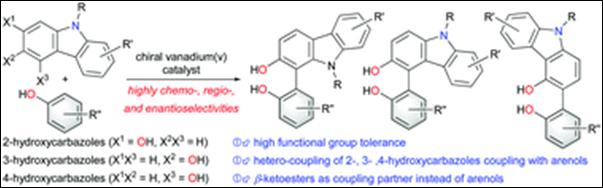
Chemo- and Enantioselective
Hetero-coupling of Hydroxycarbazoles Catalyzed by a Chiral Vanadium(v) complex
Sako,
M.; Higashida, K.; Kamble, G. T.; Kaut, K.; Kumar, A.; Hirose, Y.; Zhou, D.;
Suzuki, T.; Rueping, M.; Maegawa,
T.; Takizawa, S.; Sasai, H. Org. Chem. Front., 2021, 8, 4878 (DOI).
5.
Chiral Vanadium(v)-catalyzed Oxidative Coupling of
4-Hydroxycarbazoles
Kamble, G.; Salem, M.; Abe, T.;
Park, H.; Sako, M.; Takizawa, S.; Sasai, H. Chem. Lett., 2021,
50, 1755-1757 (DOI).
6.
Azopyridine-based
Chiral Oxazolines with Rare-earth Metals for Photoswitchable Catalysis
Chem. Commun., 2021,
57, 7414 (DOI).
7.
Energy-, Time-, and Labor-saving
Synthesis of α-Ketiminophosphonates:
Machine-learning-assisted Simultaneous Multiparameter Screening for
Electrochemical Oxidation
8. Application of an Electrochemical Microflow Reactor for Cyanosilylation: Machine Learning-assisted Exploration of Suitable
Reaction Conditions for Semi-large-scale Synthesis
, E.; , S. J. Org. Chem., 2021,86, 16035-16044 (DOI).
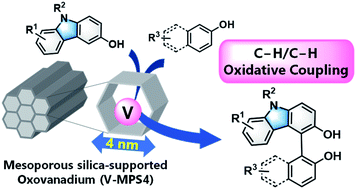
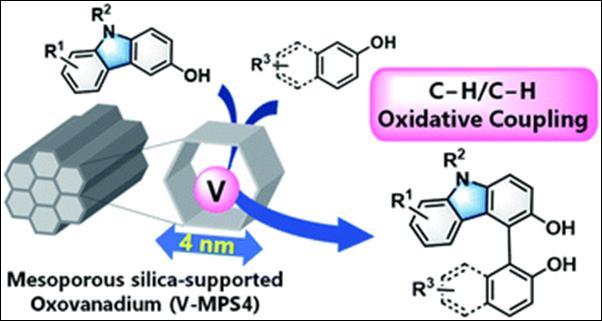
9.
Chemo- and Regioselective Cross-dehydrogenative Coupling
Reaction of 3-Hydroxycarbazoles with Arenols Catalyzed by a Mesoporous
Silica-supported Oxovanadium
Kasama, K.; Kanomata, K.; Hinami, Y.; Mizuno, K.; Uetake, Y.; Amaya, T.; Sako, M.; Takizawa, S.; Sasai, H.; Akai, S. RSC Adv. 2021, 11, 35342-35350 (DOI).
2020
Original Paper
1.
Vanadium(V) Complex-catalyzed One-pot
Synthesis of Phenanthridines via a Pictet-Spengler-Dehydrogenative Aromatization Sequence
Sako, M.; Losa, R.; Takiishi, T.; Vo-Thanh, G.; Takizawa, S.; Sasai, H. Catalysts 2020, 10, 860(DOI)
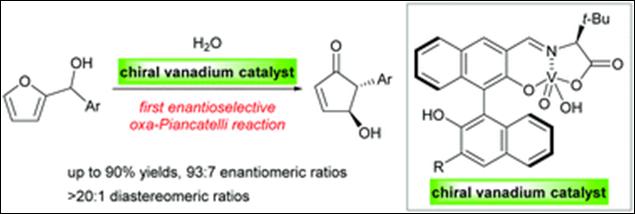
2. Catalytic
and Enantioselective oxa-Piancatelli Reaction using
Chiral Vanadium Complex
Schober,
L.; Sako, M.; Takizawa, S.; Gröger, H.;
Sasai, H. Chem. Commun. 2020, 56,
10151-10154(DOI)

3. Synthesis
of Allylamine Derivatives via
Intermolecular Aza-Wacker-Type Reaction Promoted by Palladium–SPRIX Catalyst
Sen,
A.; Zhu, L.; Takizawa, S.; Takenaka, K.; Sasai, H. Adv. Synth. Catal. 2020, 362, 3558-3563(DOI)
4.
Exploration of Flow
Reaction Conditions Using Machine-learning for Enantioselective Organocatalyzed
Rauhut-Currier and [3+2] Annulation Sequence
Kondo, M.; Wathsala, H. D. P.; Sako, M.; Hanatani, Y.; Ishikawa, K.; Hara, S.; Takaai,
T.; Washio, T.; Takizawa, S.; Sasai, H. Chem. Commun. 2020, 56, 1259-1262. (DOI)

<Highlighted in Synfacts 2020, 16, 366. (DOI)>
<本研究成果はプレスリリースされました>
EurekAlert!(https://eurekalert.org/pub_releases/2020-02/ou-trs021820.php)
AlphaGalileo(https://www.alphagalileo.org/Item-Display/ItemId/189379)
5.
Enantioselective
One-pot Synthesis of 3-Azabicyclo[3.1.0]hexanes via Allylic Substitution and
Oxidative Cyclization
Chaki, B. M.; Takenaka, K.; Zhu, L.; Tsujihara, T.; Takizawa, S.; Sasai, H. Adv.
Synth. Catal. 2020, 362(7), 1537-1547. (DOI)
<Selected as a Very Important Publication (VIP)>
<Highlighted in Synfacts 2020, 16, 781. (DOI)>
Book & Review
1.
“第三級カルボカチオンの立体制御によるエナンチオ収束型触媒的SN1反応”(Review de Debut)
佐古真,有機合成化学協会誌,Vol.78, No.1 (2020),60-61.📚
2.
Organocatalytic Synthesis of Highly Functionalized
Heterocycles by Enantioselective aza-Morita–Baylis–Hillman-type Domino Reactions
Takizawa, S. Chem. Pharm. Bull. 2020, 68, 299-315.📚
<2019年度日本薬学会学術振興賞受賞記念総説>
3. Chiral vanadium complex-catalyzed oxidative coupling of
arenols
Sako, M.; Takizawa, S.;
Sasai, H. Tetrahedron 2020, 76, 131645. (DOI)
2019
Original
Paper
1. Asymmetric
Cyclizative Dimerization of (ortho-Alkynyl Phenyl)
(Methoxymethyl) Sulfides with Palladium(II) Bisoxazoline
Catalyst
Peng, C.; Kusakabe, T.; Kikkawa, S.; Mochida, T.; Azumaya,
I.; Dhage, Y. D.; Takahashi, K.; Sasai, H.; Kato, K. Chem. Eur. J. 2019, 25(3), 733-737. (DOI)

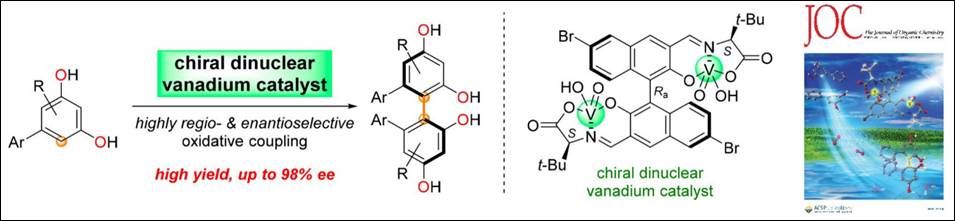
2.
Chiral Dinuclear
Vanadium Complex-mediated Oxidative Coupling of Resorcinols
Sako, M.; Aoki, T.; Zumbrägel,
N.; Schober, L.; Gröger, H.; Takizawa, S.; Sasai, H. J. Org. Chem. 2019, 84(3), 1580-1587. (DOI)
<Selected as a Cover Picture>
3. Room-Temperature, Metal-Free and One-Pot Preparation of 2H-Indazoles via Mills Reaction and Cyclization Sequence
Kondo, M.; Takizawa, S.; Jiang, Y.; Sasai, H. Chem. Eur. J. 2019, 25(42), 9866-9869. (DOI)
Book &
Review
1. キラルバナジウム触媒によるヘリセン様化合物の簡便合成
佐古 真,滝澤 忍,笹井 宏明,生産と技術 2019, 71(2), 77-80.
The Development of Efficient Synthesis for Oxahelicenes
Using Chiral Vanadium Catalyst
Sako, M.; Takizawa, S.; Sasai, H. 生産と技術 2019, 71(2), 77-80.
Original
Paper
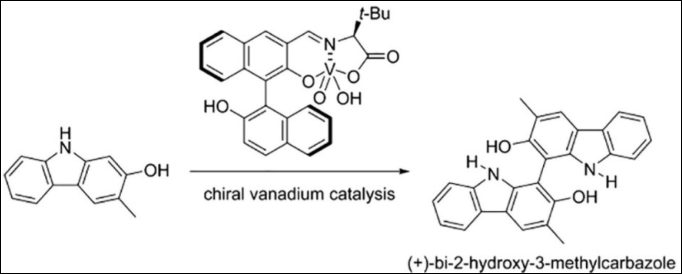
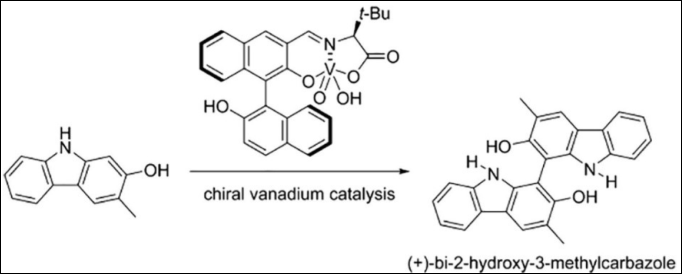
1. Asymmetric
Oxidative Coupling of Hydroxycarbazoles: Facile
Synthesis of (+)-Bi-2-hydroxy-3-methylcarbazole
Sako, M.; Sugizaki, A.; Takizawa, S. Bioorg. Med.
Chem. Lett. 2018, 28,
2751-2753. (DOI)
(Dedicated to Professor Dr. Dale L. Boger on the occasion of his 65th
birthday.)
2. Enantioselective
Synthesis of Spiro (isoxazole-isoxazoline) Hybrid
Ligand
Chaki, B. M.; Wakita, K.; Takizawa, S.; Takenaka, K.; Sasai, H. Heterocycles 2018, 97, 493–505. (DOI)
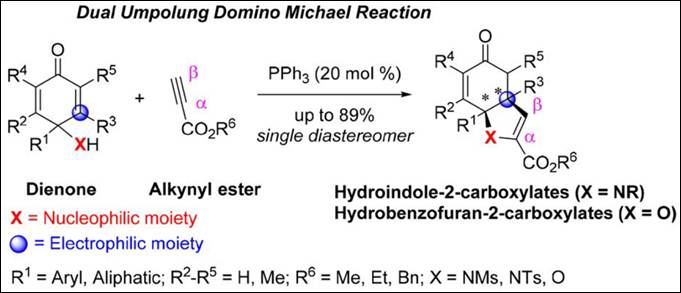
3. Phosphine-Catalyzed
Dual Umpolung Domino Michael Reaction: Facile Synthesis of Hydroindole-
and Hydrobenzofuran-2-Carboxylates
Kishi, K.; Takizawa, S.; Sasai, H. ACS Catal. 2018,
8, 5228-5232. (DOI)
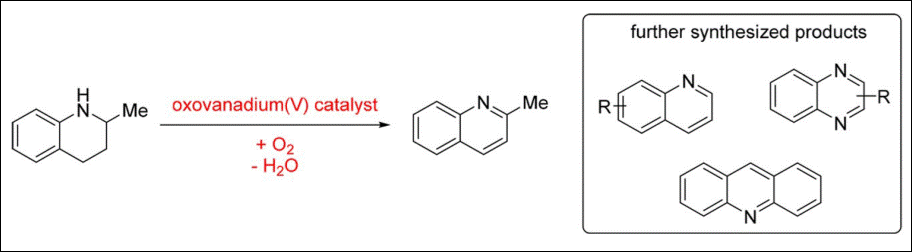
4. Vanadium-Catalyzed
Dehydrogenation of N‑Heterocycles in
Water
Zumbrägel, N.; Sako, M.;
Takizawa, S.; Sasai, H.; Gröger, H. Org. Lett. 2018, 20, 4723-4727. (DOI)
5. Enantioselective
Aza-Wacker-Type Cyclization Promoted by Pd-SPRIX Catalyst
Sen, A.; Takenaka, K.; Sasai, H. Org. Lett. 2018, 20, 6827-6831. (DOI)
Book &
Review
1. “多機能有機分子不斉触媒を用いる環境調和型ドミノ反応の開発”
「有機分子触媒の開発と工業利用」
笹井宏明、滝澤忍,シーエムシー出版 (2018) 220-232.📚
2. “ベンゼンの触媒的非対称化を鍵とするパンクラチスタチン類の不斉全合成”
ファルマシア「トピックス」
佐古真,ファルマシア, 第54巻7号 (2018),710.📚
3. “キラルバナジウム触媒を用いるエナンチオ選択的酸化カップリング反応の開発と応用”
佐古真、滝澤忍、笹井宏明,有機合成化学協会誌,Vol.76,
No.9 (2018),874-884.📚
“Chiral Vanadium Complex-catalyzed
Enantioselective Oxidative Coupling Reactions”
Sako, M.; Takizawa, S.; Sasai, H. J. Synth. Org.
Chem. Jpn. 2018,
76, 874–884. (DOI)
Original
Paper
1. Facile
Synthesis of Spirooxindoles via an Enantioselective
Organocatalyzed Sequential Reaction of Oxindoles with Ynone
Takizawa, S.;
Kishi, K.; Kusaba, M.; Bai, J.; Suzuki, T.; Sasai, H. Heterocycles 2017, 95(2), 761-767. (DOI)
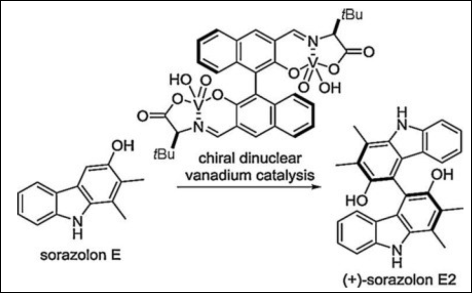
2. Short Syntheses of 4-Deoxycarbazomycin B, Sorazolon E, and (+)-Sorazolon E2
Sako, M.; Ichinose, K.; Takizawa, S.; Sasai, H. Chem. Asian J. 2017, 12(12), 1305–1308. (DOI)
3. Determination
of the Absolute Configuration of Compounds Bearing Chiral Quaternary Carbon
Centers Using the Crystalline Sponge Method
Sairenji, S.; Kikuchi, T.; Abozeid, M. A.; Takizawa, S.; Sasai,
H.; Ando, Y.; Ohmatsu, K.; Ooi, T.; Fujita, M. Chem. Sci. 2017, 8(7), 5132–5136. (DOI)
4. Enantioselective Synthesis
of Tetrahydrocyclopenta[b]indole Bearing a Chiral
Quaternary Carbon Center via Pd(II)-SPRIX-Catalyzed C–H Activation
Abozeid, M. A.; Sairenji, S.; Takizawa, S.; Fujita, M.;
Sasai, H. Chem. Commun. 2017, 53,
6887-6890. (DOI)
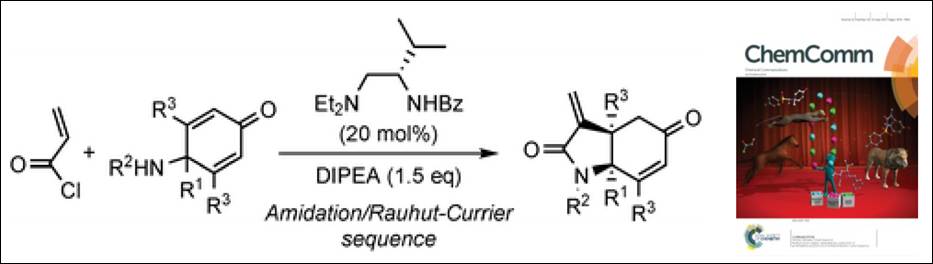
5. Multifunctional Catalysis:
Stereoselective Construction of α-Methylidene-γ-Lactams
via Amidation/Rauhut–Currier
Sequence
Kishi, K.; Arteaga, F. A.; Takizawa, S.; Sasai, H. Chem. Commun. 2017,
53. 7724-7727. (DOI)
<Selected as an
Inside Front Cover>
6. Reversal of
Enantioselectivity Approach to BINOLs via Single and Dual 2‑Naphthol Activation
Modes
Kim, H. Y.; Takizawa, S.; Sasai, H.; Oh, K. Org. Lett. 2017, 19(14),
3867-3870. (DOI)
7. Enantio- and
Diastereoselective Betti/aza-Michael Sequence: Single
Operated Preparation of Chiral 1,3-Disubstituted Isoindolines
Takizawa, S.; Sako, M.; Abozeid, M. A.; Kishi, K.;
Wathsala, H. D. P.; Hirata, S.; Murai, K.; Fujioka, H.; Sasai, H. Org. Lett. 2017, 19(19), 5426-5429. (DOI)
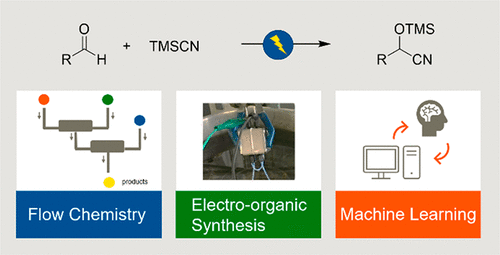
8. Chiral Organocatalyzed
Intermolecular Rauhut–Currier Reaction of Nitroalkenes with Ethyl Allenoate
Takizawa, S.; Sako, M.; Kishi, K.; Shigenobu, M.;
Vo-Thanh, G.; Sasai, H. Chem. Pharm. Bull. 2017, 65, 997-999. (DOI)
<Selected as a Cover Picture>
Book &
Review
1. 多機能有機分子触媒を用いるエナンチオ選択的ドミノ反応の開発
滝澤忍、笹井宏明,化学工業,第68巻9号 (2017), 31-38.
Original
Paper
1. Enantioselective
Organocatalytic Oxidation of Ketimines
Takizawa, S.; Kishi, K.; Abozeid, M. A.; Murai, K.; Fujioka, H.; Sasai, H. Org. Biomol. Chem. 2016, 14, 761-767. (DOI)
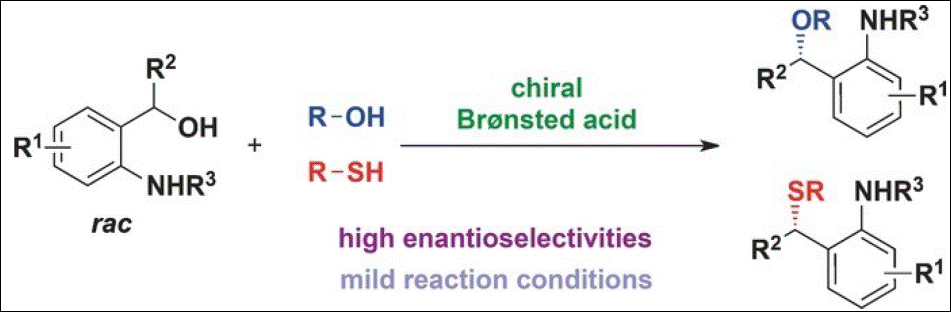
2. Asymmetric Brønsted Acid Catalyzed
Substitution of Diaryl Methanols
with Thiols and Alcohols for the Synthesis of Chiral Thioethers and Esters
Chatupheeraphat, A.; Liao, H.-H.; Mader, S.; Sako, M.; Sasai, H.; Atodiresei,
I.; Rueping, M. Angew. Chem. Int. Ed. 2016, 55, 4803-4807. (DOI)
3. Efficient Enantioselective Synthesis of Oxahelicenes Using Redox/Acid Cooperative Catalysts
Sako, M.; Takeuchi, Y.; Tsujihara, T.; Kodera, J.;
Kawano, T.; Takizawa, S.; Sasai, H. J. Am. Chem. Soc. 2016, 138(36),
11481-11484. (DOI)
4. Organocatalyzed [4+2] Annulation of
All-Carbon Tetrasubstitued Alkenes with Allenoate:
Synthesis of Highly Functionalized 2H,
and 4H-Pyran Derivatives
Ngo,
T.-Thuy-Duong ; Kishi, K.; Sako, M.; Shigenobu, M.; Bournaud, C.; Toffano, M.;
Guillot, R.; Baltaze, J.-P.; Takizawa, S.; Sasai, H.;
Vo-Thanh, G. ChemistrySelect 2016,
1(17), 5414-5420. (DOI)
Book &
Review
1. “有機分子触媒を用いる脱古典的ドミノ反応の開発動向”
「有機分子触媒の化学 モノづくりのパラダイムシフト」(日本化学会 編)
滝澤忍,化学同人 (2016) 206-207.
2. A Career in Catalysis:
Masakatsu Shibasaki
Kumagai. N.; Kanai, M.; Sasai, H. ACS catal. 2016, 6(7),
4699-4709. (DOI)
Original
Paper
1. Enantioselective
Construction of C2-Symmetric Spiro Skeleton through
Intramolecular Copper-Catalyzed N-Arylation
Takenaka, K.; Sako, M.; Takatani,
S.; Sasai, H.
ARKIVOC 2015, ii, 52-63. (DOI)
2. Pd-Catalyzed Enantioselective Intramolecularα-Arylation ofα-Substituted Cyclic Ketones: Facile Synthesis of Functionalized Chiral Spirobicycles
Fan, L.; Takizawa, S.; Takeuchi, Y.; Takenaka, K.; Sasai, H. Org. Biomol. Chem. 2015, 13, 4837-4840. (DOI)
3. Palladium(II)-Catalyzed
Intramolecular Carboxypalladation–Olefin Insertion Cascade: Direct Access to Indeno[1,2,-b]furan-2-ones
Vinoth, P.; Vivekanand, T.; Suryavanshi, P. A.; Menendez, J. C.; Sasai, H.;
Sridharan, V. Org. Biomol. Chem. 2015, 13, 5175-5181. (DOI)
4. Structural Features and Asymmetric Environment of i-Pr-SPRIX Ligand
Takenaka, K.; Lin, X.; Takizawa, S.; Sasai, H. Chirality, 2015,
27, 532-537.. (DOI)
5. Enantioselective and
Aerobic Oxidative Coupling of 2-Naphthol Derivatives Using Chiral Dinuclear Vanadium(V) Complex in Water
Sako, M.; Takizawa, S.; Yoshida, Y.; Sasai, H. Tetrahedron: Asymmetry 2015, 26, 613-616. (DOI)
6. Pd(II)-Catalyzed
Diastereoselective and Enantioselective Domino Cyclization/Cycloaddition
Reactions of Alkenyl Oximes for Polycyclic Heterocycles with Four Chiral Stereogenic Centers
Abozeid, M. A.; Takizawa, S.; Sasai, H. Tetrahedron Lett. 2015, 56, 4316-4319. (DOI)
7. Palladium-Catalyzed Direct
C–H
Arylation of Isoxazoles at the 5-Position
Shigenobu, M.; Takenaka, K.; Sasai, H. Angew. Chem. Int. Ed. 2015, 54, 9572-9576. (DOI)
8. An Enantioselective Organocatalyzed aza-Morita-Baylis-Hillman
Reaction of Isatin-derived Ketimines
with Acrolein
Yoshida, Y.; Sako, M.; Kishi, K.; Sasai, H.;
Hatakeyama, S.; Takizawa, S. Org. Biomol. Chem. 2015, 13, 9022-9028. (DOI)
9. One-Pot
Catalysis Using a Chiral Iridium Complex/Bronsted Base: Catalytic Asymmetric
Synthesis of Cataponol
Suzuki, T.; Ismiyarto; Ishizaka, Y.; Zhou, D.-Y.;
Asano, K.; Sasai, H. Org.
Lett. 2015, 17, 5176-5179. (DOI)
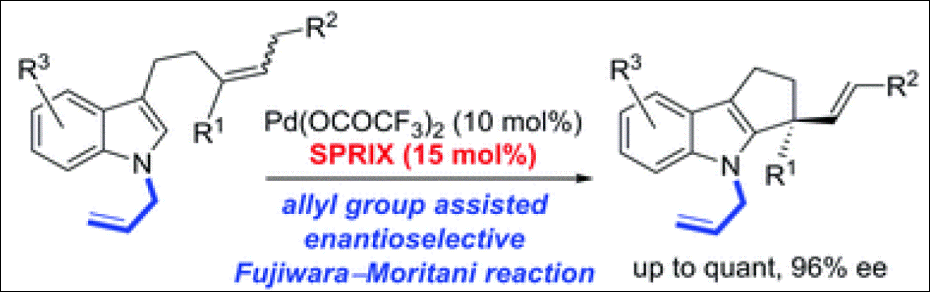
10. Phosphine-Catalyzed β,γ-Umpolung Domino
Reaction of Allenic Esters: Facile Synthesis of Tetrahydrobenzofuranones Bearing a Chiral Tetrasubstituted
Carbon Stereogenic Center
Takizawa, S.;
Kishi, K.; Yoshida, Y.; Mader, S.; Arteaga, F. A.; Lee, S.; Hoshino, M.; Rueping, M.; Fujita, M.; Sasai, H. Angew. Chem. Int. Ed. 2015, 54, 15511-15515. (DOI)
<Highlighted
in Synfacts
2016, 12, 129>
Book &
Review
1. Vanadium in Asymmetric
Synthesis: Emerging Concepts in Catalyst Design and Applications
Takizawa, S.; Gröger, H.; Sasai, H. Chem. Eur.
J. 2015, 21, 8992-8997. (DOI)
2. パラジウム2価/4価触媒サイクルを経るアルケン・アルキンの1,2−二官能基化反応
竹中和浩, 有機合成化学協会誌, 第73巻10号 (2015), 964-976.
3. Sasai, H.; Takizawa, S. “Vanadium
and Niobium-catalyzed Enantioselective Reactions” In Sustainable
Catalysis: With Non-endangered Metals, Part 1, North, M. Ed, Royal
Society of Chemistry; UK, 2015; pp 216-249.
4. パラジウム(II)錯体の反応性制御 新規エナンチオ選択的パラジウム触媒反応の開発
竹中和浩, 化学と工業, 第68巻12号 (2015),
1123-1124.
Original
Paper
1. Enantioselective
Oxidative-Coupling of Polycyclic Phenols
Takizawa, S.; Kodera, J.; Yoshida, Y.; Sako,
M.; Breukers, S.; Enders, D.; Sasai, H. Tetrahedron 2014, 70, 1786-1793. (DOI)
2. Enantioselective
Organocatalyzed Formal [4+2] Cycloaddition of Ketimines
with Allenoates: Easy Access to a Tetrahydropyridine Framework with a Chiral
Tetrasubstituted Stereogenic Carbon Center
Takizawa, S.; Arteaga, F. A.; Yoshida, Y.; Suzuki, M.; Sasai,
H. Asian J. Org. Chem. 2014, 3, 412-415. (DOI)
3. Palladium Enolate
Umpolung: Cyclative Diacetoxylation of Alkynyl Cyclohexadienones Promoted by a Pd/SPRIX Catalyst
Takenaka, K.; Mohanta, S. C.; Sasai, H. Angew. Chem. Int. Ed. 2014, 53, 4675-4679. (DOI)
<Highlighted
in Synfacts
2014, 10, 619>
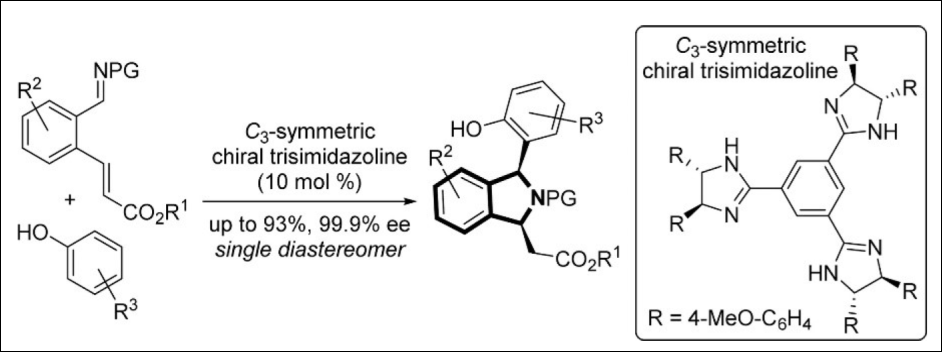
4. C3-Symmetric Chiral Trisimidazoline-catalyzed Friedel–Crafts (FC)-type
Reaction
Takizawa, S.; Hirata, S.; Murai, K.; Fujioka, H.; Sasai, H. Org. Biomol. Chem. 2014, 12, 5827-5830. (DOI)
5. Facile Regio- and
Stereoselective Metal-Free Synthesis of All-Carbon Tetrasubstituted Alkenes
Bearing a C(sp3)−F Unit via Dehydroxyfluorination of Morita−Baylis−Hillman (MBH) Adducts
Takizawa, S.; Arteaga, F. A.; Kishi, K.; Hirata,
S.; Sasai, H. Org. Lett. 2014, 16, 4162-4165. (DOI)
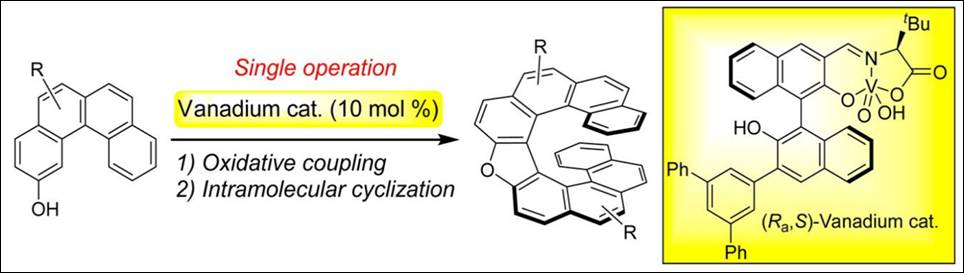
Book &
Review
1. Takizawa, S.; Sasai, H. “Metal-catalyzed
Enantio- and Diastereoselective C–C Bond-forming
Reactions in Domino Processes” In Domino Reactions:
Concepts for Efficient Organic Synthesis, Tietze, L. F. Ed, Wiley-VCH Verlag
GmbH & Co. KGaA; Chapter 11, pp. 419-462 (2014).
2. Takizawa, S.; Sasai, H.
"Enantioselective Acid-Base Organocatalyzed Domino Reactions Based on aza-Morita-Baylis-Hillman Process", J. Synth. Org.
Chem. Jpn., vol. 72, No. 7, pp. 781-796
(2014).
3. Sasai, H. “The Henry
(Nitroaldol) Reaction” In Comprehensive Organic
Syntheses, Second Edition, Knochel, P.; Molander, G. A. Eds, Elsevier;
Amsterdam; Volume 2, Chapter 2-13, pp 543-570 (2014).
4. Takenaka, K; Sasai, H. “Addition Reactions
with Formation of Carbon–Oxygen Bonds: (iv) The Wacker
Oxidation and Related Reactions” In Comprehensive
Organic Syntheses, Second Edition, Knochel, P.; Molander, G. A. Eds, Elsevier;
Amsterdam; Volume 7, Chapter 7-17, pp 431-491 (2014).
Original
Paper
1. Facile Synthesis of α-Methylidene-γ-Butyrolactones: Intramolecular Rauhut-Currier Reaction Promoted by
Chiral Acid-Base Organocatalysts
Takizawa, S.; Nguyen, T. M.-N.; Grossmann, A.; Suzuki, M.; Enders, D.; Sasai H.
Tetrahedron 2013,
69, 1202-1209. (DOI)
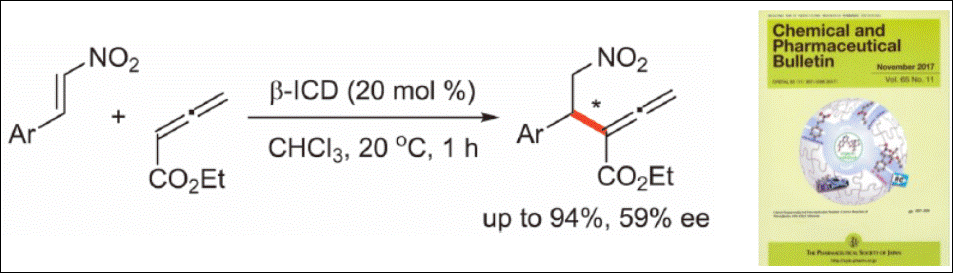
2. Vanadium-Catalyzed
Enantioselective Friedel-Crafts-Type Reactions
Takizawa, S.; Arteaga, F. A.; Yoshida, Y.; Kodera, J.;
Nagata, Y.; Sasai, H. Dalton Trans. 2013, 42,
11787-11790. (DOI)
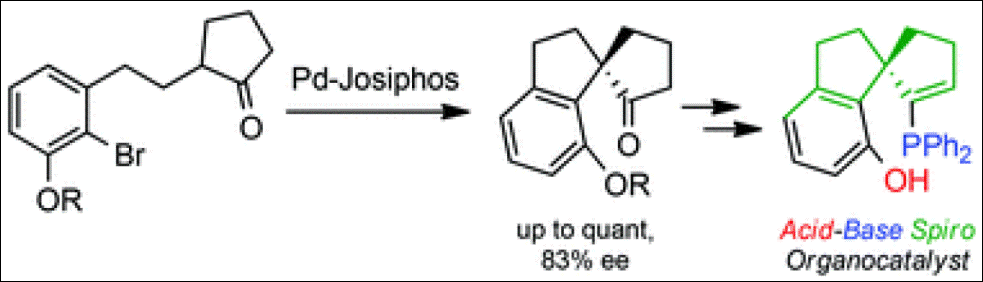
3. DFT
Study on 5-Endo-Trig-Type Cyclization of 3-Alkenoic Acids Using Pd–SPRIX Catalyst: Importance of the Rigid Spiro Framework for Both
Selectivity and Reactivity
Gabr, R. K. M.; Hatakeyama, T.; Takenaka, K.; Takizawa, S.; Okada, Y.;
Nakamura, M.; Sasai, H. Chem. Eur. J. 2013, 19,
9518-9525. (DOI)
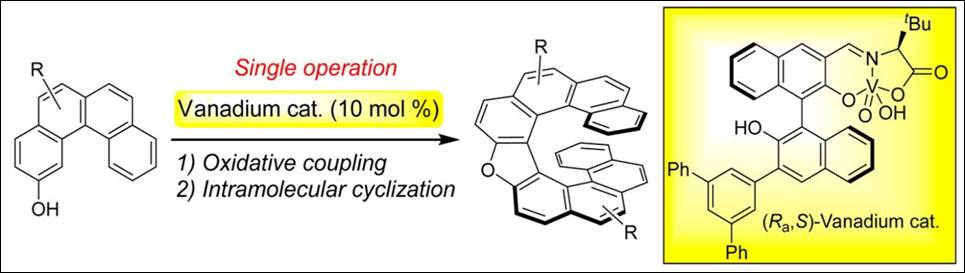
4. o-(Hydroxyalkyl)
P-Chirogenic Phosphines as Functional Chiral Lewis
Bases
Rémond, E.; Bayardon,
J.; Takizawa, S.; Rousselin, Y.; Sasai, H.; Jugé,
S. Org. Lett. 2013, 15, 1870-1873. (DOI)
5. Carbonylation of Propargyl Carbamates with Palladium(II) Bisoxazoline Catalysts: Efficient Synthesis of
5-Methyl-3(2H)-furanones
Kusakabe, T.; Takahashi,
T.;
Shen, R.; Ikeda, A.; Dhage, Y. D.; Kanno, Y.; Inouye, Y.;
Sasai, H.; Mochida, T.;
Kato, K.
Angew. Chem. Int. Ed. 2013, 52, 7845-7849. (DOI)
6. Enantioselective
Multicatalytic Synthesis of α-Benzyl-β-hydroxyindan-1-ones
Suzuki,
T.; Ishizaka, Y.; Ghozati, K.; Zhou, D.-Y.; Asano,
K.; Sasai, H. Synthesis 2013,
45, 2134-2136. (DOI)
7. Pd(II)-SDP-Catalyzed Enantioselective 5-Exo-Dig Cyclization of γ-Alkynoic Acids: Application to the
Synthesis of Functionalized Dihydrofuran-2(3H)-ones Containing a Chiral
Quaternary Carbon Center
Sridharan,
V.; Fan, L.; Takizawa, S.; Suzuki, T.; Sasai, H. Org. Biomol. Chem. 2013, 11, 5936-5943. (DOI)
8. P-Chirogenic Organocatalysts: Application to the aza-Morita-Baylis-Hillman (aza-MBH)
Reaction of Ketimines
Takizawa, S.;
Rémond, E.;
Arteaga, F. A.; Yoshida, Y.; Sridharan, V.; Bayardon, J.;
Jugé
S.; Sasai, H. Chem. Commun. 2013, 49, 8392-8394. (DOI)
<Highlighted
in Synfacts
2013, 9, 1297>
9. Organocatalyzed Formal [2+2] Cycloaddition of Ketimines with Allenoates: Facile Access to Azetidines with
a Chiral Tetrasubstituted Carbon Stereogenic Center
Takizawa, S.; Arteaga, F. A.; Yoshida, Y.; Suzuki, M.; Sasai, H. Org. Lett. 2013,
15, 4142-4145. (DOI)
10. Chiral Bifunctional Organocatalysts Bearing a
1,3-Propanediamine Unit for the aza-MBH Reaction
Hirata, S.; Tanaka, K.; Matsui, K.; Arteaga, F. A.; Yoshida, Y.; Takizawa, S.;
Sasai, H. Tetrahedron: Asymmetry 2013,
24, 1189-1192. (DOI)
11. Enantioselective
Pd(II)/Pd(IV) Catalysis Utilizing SPRIX Ligand: Efficient Construction of
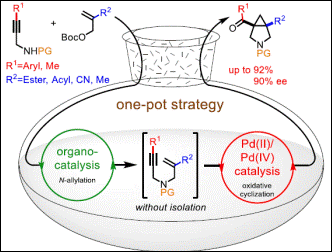
Chiral 3-Oxy-Tetrahydrofurans
Takenaka, K.;
Dhage, Y. D.; Sasai, H. Chem. Commun. 2013, 49, 11224-11226. (DOI)
<Highlighted
in Synfacts
2014, 10, 275>
Original
Paper
1. Enantioselective
Synthesis of a-Alkylidene-g-Butyrolactones:
Intramolecular Rauhut-Currier Reaction Promoted by Acid/Base Organocatalysts
Takizawa, S.; Nguyen, T. M.-N.; Grossmann, A.; Enders, D.; Sasai H. Angew. Chem., Int. Ed. 2012,
51, 5423-5426. (DOI)
<Highlighted
in Synfacts
2012, 8, 787>
2. Design and Synthesis of
Spiro Bis(1,2,3-triazolium) Salts As Chiral Ionic Liquids
Yoshida, Y.; Takizawa, S.; Sasai, H. Tetrahedron: Asymmetry 2012, 23, 843-851. (DOI)
Book &
Review
1. Sasai, H. “Direct C-C Bond Formation
(Henry, Aza-Henry)” in Comprehensive Chirality,
Carreira, E. M. and Yamamoto, H. Ed., Elsevier B. V.; Amsterdam, 4, pp. 214-242
(2012).
2. Sasai, H. and Takizawa, S. “C-C Bond Formation: (aza)
Morita-Baylis-Hillman Reaction” in Comprehensive
Chirality, Carreira, E. M. and Yamamoto, H. Ed., Elsevier B. V.; Amsterdam, 6,
pp. 234-263 (2012).
Original
Paper
1. An
Enantioselective Organocatalyzed aza-MBH Domino
Process: Application to the Facile Synthesis of Tetrahydropyridines
Takizawa, S.; Inoue, N.; Sasai H. Tetrahedron Lett. 2011, 52, 377-380. (DOI)
2. Pd(II)-SPRIX Catalyzed
Enantioselective Construction of Pyrrolizines/Pyrroloindoles Employing Molecular Oxygen As the Sole
Oxidant
Ramalingan, C.; Takenaka, K.; Sasai, H. Tetrahedron 2011, 67, 2889-2894. (DOI).
3. Chlorinative Cyclization of 1,6-Enynes
by Enantioselective Palladium(II)/Palladium(IV) Catalysis
Takenaka, K.; Hashimoto, S.; Takizawa, S.; Sasai, H. Adv. Synth. Catal. 2011, 353,
1067-1070. (DOI).
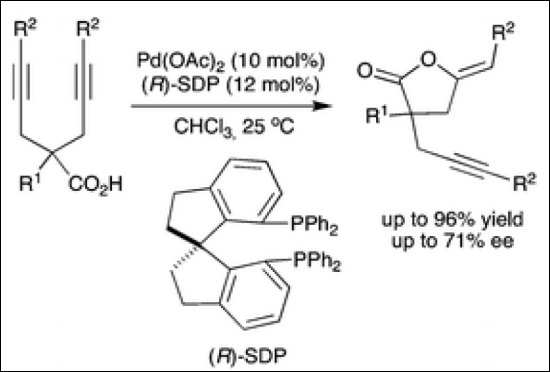
4. Enantioselective
Cyclization of 4-Alkenoic Acids via an Oxidative Allylic C-H Esterification
Takenaka, K.; Akita, M.; Tanigaki, Y.; Takizawa, S.; Sasai, H. Org. Lett. 2011, 13,
3506-3509. (DOI).
<Highlighted
in Synfacts 2011, 991>
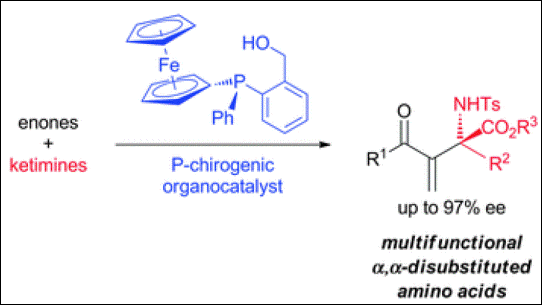
5. A Bifunctional Spiro-Type Organocatalyst with High Enantiocontrol: Application to the
Aza-Morita-Baylis-Hillman Reactions
Takizawa, S.; Kiriyama, K.; Ieki, K.; Sasai, H. Chem. Commun. 2011, 47,
9227-9229. (DOI).
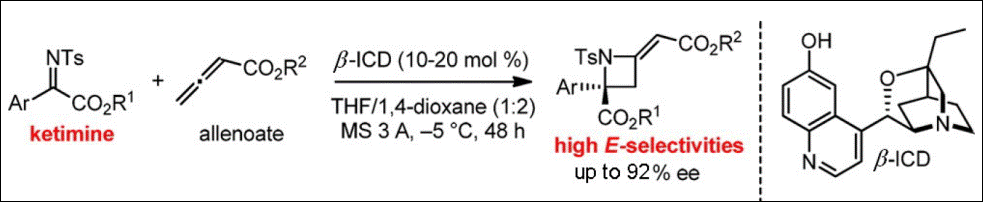
6. Synthesis of Spiro
Bis(1,2,3-triazolium) Salts As Chiral Ionic Liquids
Yoshida, Y.; Takizawa, S.; Sasai, H. Tetrahedron Lett. 2011, 52, 6877-6879. (DOI).
Book &
Review
1.
Immobilization of Multicomponent Asymmetric
Catalysts (MACs)
「Polymeric Chiral Catalyst Design and Chiral Polymer Synthesis」(Itsuno, S. Ed.)
Takizawa, S.; Sasai, H. John Wiley & Sons, (2011) 293-322.
Original
Paper
1. Asymmetric Synthesis of
Chiral Spiro Bis(isoxazoline) and Spiro (Isoxazole-Isoxazoline) Ligands
Takenaka, K.; Nagano, T.; Takizawa, S.; Sasai, H. Tetrahedron:
Asymmetry 2010, 21, 379-381. (DOI)
2. Enantioselective
6-Endo-Trig Wacker-Type Cyclization of 2-Geranylphenols: Application to Facile
Synthesis of (−)-Cordiachromene
Takenaka, K.; Tanigaki, Y.; Patil, M. L.; Rao, C. V. L.; Takizawa, S.; Suzuki, T.; Sasai, H. Tetrahedron: Asymmetry 2010, 21, 767-770. (DOI)
3. Acid-Base
Organocatalysts for the Aza-Morita-Baylis-Hillman Reaction of Nitroalkenes
Takizawa, S.; Horii, A.; Sasai, H. Tetrahedron: Asymmetry 2010, 21, 891-894.
(DOI)
4. Enantioselective
Wacker-Type Cyclization of 2-Alkenyl-1,3-Diketones Promoted by Pd-SPRIX
Catalyst
Takenaka, K.; Mohanta, S. C.; Patil, M. L.; Rao, C. V. L.; Takizawa, S.;
Suzuki, T.; Sasai, H. Org. Lett. 2010, 12, 3480-3483. (DOI)
<Highlighted
in Synfacts 2010, 1263>
5. Formal Total Synthesis of Ottelione Using Iridium-Catalyzed Oxidative Desymmetrization
Suzuki, T.; Ghozati, K.; Zhou, D.-Y.; Katoh, T.;
Sasai, H. Tetrahedron 2010, 66, 7562-7568. (DOI)
6. Enantioselective
Synthesis of Isoindolines: Organocatalyzed Domino
Process Based on the aza-Morita-Baylis-Hillman (aza-MBH) Reaction
Takizawa, S.; Inoue, N.; Hirata, S.; Sasai H. Angew. Chem. Int.
Ed. 2010, 49, 9725-9729. (DOI)
<Highlighted
in Synfactcs 2011, 221>
7. Pd-Catalyzed
5-Endo-Trig-Type Cyclization of b,g-Unsaturated Carbonyl
Compounds: an Efficient Ring Closing Reaction to Give g-Butenolides and
3-Pyrrolin-2-ones
Bajracharya, G. B.; Koranne, P. S.; Nadaf, R. N.; Gabr, R. K. M.; Takenaka, K.;
Takizawa, S.; Sasai H. Chem. Commun. 2010, 46, 9064-9066. (DOI)
Book &
Review
1.
「使える!有機合成反応241実践ガイド」(丸岡啓二, 野崎京子, 石井康敬, 大寺純蔵, 富岡清 編著)
笹井宏明, 滝澤忍, 竹中和浩, 化学同人 (2010) 224-225, 262-263, 280-281,
360-361.
Original
Paper
1.
Dicationic Palladium(II)-Spiro bis(isoxazoline) Complex
for Highly Enantioselective Isotactic Copolymerization of CO with Styrene
Derivatives
Bajracharya, G. B.; Koranne, P. S.; Tsujihara, T.; Takizawa, S.;
Onitsuka, K.; Sasai, H. Synlett 2009, 310-314. (DOI)
2.
PdII/PdIV Catalytic Enantioselective
Synthesis of Bicyclo[3.1.0]hexanes via Oxidative
Cyclization of Enynes
Tsujihara, T.; Takenaka, K.; Onitsuka,
K.; Hatanaka, M.; Sasai, H. J. Am. Chem. Soc. 2009, 131, 3452-3453. (DOI)
<Highlighted
in Synfacts 2009, 635>
3.
Enantioselective Synthesis
of C2-Symmetric Spirobilactams via Pd-Catalyzed Intramolecular Double N-Arylation
Takenaka, K.; Itoh, N.;
Sasai, H. Org. Lett. 2009, 11, 1483-1486. (DOI)
<Highlighted
in Synfacts 2009, 602>
4.
Development of Chiral Spiro Ligands for
Metal-Catalyzed Asymmetric Reactions
Bajracharya, G. B.; Arai, M. A.; Koranne, P. S.; Suzuki, T.; Takizawa, S.;
Sasai, H. Bull.
Chem. Soc. Jpn. 2009, 82, 285-302. (DOI)
5. One-pot Preparation of
Chiral Dinuclear Vanadium(V) Complex
Takizawa, S.; Rajesh, D.; Katayama, T.; Sasai, H. Synlett 2009,
1667-1669. (DOI)
6. Regio- and
Enantioselective Allylation of Indole Catalyzed by a Planar-chiral
Cyclopentadienyl-Ruthenium Complex
Onitsuka, K.; Kameyama, C.; Sasai, H. Chem. Lett. 2009, 38, 444-445.
(DOI)
7. Ir-Catalyzed Oxidative Desymmetrization of meso-Diols
Suzuki, T.; Ghozati, K.; Katoh, T.; Sasai, H. Org. Lett. 2009, 11, 4286-4288. (DOI)
<Highlighted
in Synfacts 2010, 76>
8. Enantioselective
Intramolecular Oxidative Aminocarbonylation of Alkenylureas Catalyzed by Palladium-Spiro Bis(isoxazoline) Complexes
Tsujihara, T.; Shinohara, T.; Takenaka, K.; Takizawa, S.; Onitsuka, K.;
Hatanaka, M.; Sasai, H. J. Org. Chem. 2009, 74,
9274-9279. (DOI)
Book &
Review
1.
“第10章 酸塩基複合型触媒 アザMBH反応を中心として”
「化学フロンティア21 進化を続ける有機触媒−有機合成を革新する第三の触媒」 (丸岡啓二 編)
笹井宏明, 滝澤忍, 化学同人 (2009) 120-127.
2.
複数の構成要素を持つ不斉触媒“Multicomponent Asymmetric Catalyst (MAC)”の固定化
滝澤忍, 荒井孝義, 笹井宏明, 有機合成化学協会誌, 第67巻3号 (2009), 194-207.
3.
Development of Dinuclear Vanadium
Catalysts for Enantioselective Coupling of 2-Naphthols via a Dual Activation
Mechanism
Takizawa, S. Chem. Pharm. Bull. 2009, 57,
1179-1188.
4.
二重活性化能を有する酸塩基型不斉有機分子触媒の開発とaza-Morita-Baylis-Hillman反応への展開
滝澤忍, 薬学雑誌, 第129巻10号 (2009), 1201-1210.
Original Paper
1.
Regio- and Enantioselective
O-Allylation of Phenol and Alcohol
Catalyzed by a Planar-Chiral Cyclopentadienyl Ruthenium Complex
Onitsuka, K.; Okuda, H.; Sasai, H. Angew. Chem. Int.
Ed. 2008, 47, 1454-1457. (DOI)
<Highlighted
in Synfacts 2008, 380>
2.
Dual Activation in Oxidative Coupling of
2-Naphthols Catalyzed by Chiral Dinuclear Vanadium
Complexes
Takizawa, S.; Katayama, T.; Somei, H.; Asano, Y.;
Yoshida, T.; Kameyama, C.; Rajesh, D.; Onitsuka, K.; Suzuki, T.; Mikami, M.; Yamataka, H.; Jayaprakash, D.; Sasai, H. Tetrahedron 2008,
64, 3361-3371. (DOI)
3.
Chiral Dinuclear
Vanadium(V) Catalysts for Oxidative Coupling of 2-Naphthols
Takizawa, S.; Katayama, T.; Kameyama, C.; Onitsuka, K.; Suzuki, T.; Yanagida,
T.; Kawai, T.; Sasai, H. Chem. Commun. 2008, 1810-1812. (DOI)
<Highlighted
in Synfacts 2008, 737>
4.
Divergent Synthesis of Chiral Spiro
(Isoxazole-Isoxazoline) Hybrid Ligands
Takenaka, K.; Nakatsuka, S.; Tsujihara, T.; Koranne, P. S.; Sasai, H. Tetrahedron: Asymmetry 2008,
19, 2492-2496. (DOI)
5.
Recent Development on Chiral Ionic Liquids: Design,
Synthesis, and Applications
Patil, M. L.; Sasai, H. Chem. Rec. 2008, 8, 98-108. (DOI)
6.
Dinuclear Chiral
Vanadium Catalysts for Oxidative Coupling of 2-Naphthols via a Dual Activation
Mechanism
Takizawa, S.; Katayama, T.; Sasai, H. Chem. Commun. 2008, 4113-4122. (DOI)
Book &
Review
1.
「Asymmetric Phase Transfer Catalysis」(Maruoka, K. Eds.)
Sasai, H.; Patil, M. L. Wiley-VCH,
(2008) 135-159.
Original Paper
1.
Synthesis
of Novel Spiro Imidazolium Salts as Chiral Ionic Liquids
Patil, M. L.; Rao, C. V. L.; Takizawa, S.; Takenaka, K.; Onitsuka, K.; Sasai,
H. Tetrahedron 2007,
63, 12702-12711. (DOI)
2.
Development
of New Methods towards Efficient Immobilization of Enantioselective Catalysts
Takizawa, S.; Patil, M. L.; Marubayashi, K.; Sasai,
H. Tetrahedron 2007,
63, 6512-6528. (DOI)
3.
Design and Synthesis of Chiral Hybrid Spiro
(isoxazole-isoxazoline) Ligands
Koranne, P. S.; Tsujihara, T.; Arai, M. A.; Bajracharya, G. B.; Suzuki, T.;
Onitsuka, K.; Sasai, H. Tetrahedron: Asymmetry 2007, 18, 919-923. (DOI)
4.
Enantioselective Glyoxylate-ene Reaction using a Novel Spiro Bis(isoxazoline)
Ligand in Copper Catalysis
Wakita, K.; Bajracharya, G. B.; Arai, M. A.; Takizawa, S.; Suzuki, T.; Sasai,
H. Tetrahedron: Asymmetry 2007, 18, 372-376. (DOI)
5.
Optical Resolution of Tetra Isopropyl-substituted
Spiro Bis(isoxazoline) i-Pr-SPRIX
Takizawa, S.; Yogo, J.; Tsujihara, T.; Onitsuka, K.; Sasai, H. J. Organomet. Chem. 2007, 692, 495-498. (DOI)
6.
Novel Azalides Derived from Sixteen-membered
Macrolides. I. Isolation of the Mobile Dialdehyde and its One-pot
Macrocyclization with an Amine
Miura, T.; Natsume, S.; Kanemoto, K.; Atsumi, K.; Fushimi, H.; Sasai, H.; Arai,
T.; Yoshida, T.; Ajito. K. J. Antibiot. 2007, 60, 407-435.
7.
フッ素アパタイトを利用した環境に優しい無溶媒触媒反応
市原潤子, 材料の科学と工学 2007, 44, 157-162.
8.
Bifunctional Organocatalysts for
Enantioselective Aza-Morita-Baylis-Hillman (Aza-MBH) Reactions
Takizawa, S.; Matsui, K.; Sasai, H. J. Synth. Org. Chem. Jpn. 2007, 65 (11),
1089-1098.
9.
酸−塩基型不斉有機分子触媒を用いるaza-Morita-Baylis-Hillman反応
笹井宏明, 滝澤忍, 松井嘉津也, THE CHEMICAL TIMES KANTO CHEMICAL CO.
INC. 2007, 1, 3-8.
Patent
1.
「固相系酸化反応システム」
市原潤子, 山口俊郎, 特願2007-022073
Original Paper
1.
Design and Synthesis of Novel Chiral Spiro
Ionic Liquids
Patil, M. L.; Rao, C. V. L.; Yonezawa, K.; Takizawa, S.; Onitsuka, K.; Sasai,
H. Org. Lett. 2006, 8, 227-230. (DOI)
2.
Conformational Lock in Brfnsted Acid - Lewis Base Organocatalyst for the aza-Morita-Baylis-Hillman
Reaction
Matsui, K.; Tanaka, K.; Horii, A.; Takizawa, S.; Sasai, H. Tetrahedron: Asymmetry 2006,
17, 578-583. (DOI)
3.
A Brfnsted Acid - Lewis Base Organocatalyst for the aza-Morita-Baylis-Hillman
Reaction
Matsui, K.; Takizawa, S.; Sasai, H. Synlett 2006,
761-765. (DOI)
4.
Development of Efficient Methods for the Immobilisation of Multicomponent Asymmetric Catalysts
Jayaprakash, D.; Takizawa, S.; Arai, T.; Sasai, H. J. Experimental
Nanoscience 2006, 1, 477-510. (DOI)
5.
Vanadomolybdophosphoric
Acid/Fluorapatite Solid-phase System for Aerobic Oxidative Dehydrogenation
Iteya, K.; Ichihara, J.; Sasaki, Y.; Ito, S. Catal. Today 2006, 111, 349-353.
6.
Participation of New Active Species in
Epoxidation with Cetylpyridinium Dodecatungstate/FAp/Urea-H2O2 System
Ichihara, J.; Sasaki, Y. Catal. Today 2006, 117, 120-125.
Book &
Review
1.
“第18章 酸−塩基型不斉有機分子触媒によるaza-Morita-Baylis-Hillman反応”
「有機分子触媒の新展開」(柴崎正勝 監修)
笹井宏明, 滝澤忍, 松井嘉津也, シ−・エム・シー出版 (2006) 231-242.
Patent
1.
「光学活性スピロビスイソオキサゾリン誘導体とその製造方法およびその金属錯体を用いた不斉触媒反応」
笹井宏明, 脇田和彦, 加藤孝浩, 荒井緑, 特開2006-76939
2.
「光学活性スピロビスイソオキサゾール誘導体およびその製造法、並びにその金属錯体を用いた不斉触媒反応」
笹井宏明, 脇田和彦, 加藤孝浩, 荒井緑, 特開2006-76915
3.
「スピロキラリティを有する第4級アンモニウム塩およびその製造法、並びに該アンモニウム塩を用いた不斉触媒反応」
下元愛, 米澤浩司, 滝澤忍, 笹井宏明, 特開2006-76911
4.
「新規スピロ構造化合物とその製造法」
マヘッシュ エル パティル, シラムコッティ ベンカット ラクシュマン ラオ, 滝澤忍, 笹井宏明, 特開2006-76887
5.
「スピロ骨格を持つキラルな相間移動触媒およびその製造法、並びにそれを用いた不斉触媒反応」
米澤浩司, 下元愛, 滝澤忍, 笹井宏明, 特開2006-70001
6.
「Novel organic molecular catalyst having
binaphthol skeleton and processes for producing the same and application
thereof」
Sasai Hiroaki, Takizawa Shinobu, Matsui Katsuya, Patent No. US 2006-009646
7.
「ビナフトール骨格を有する新規有機分子触媒およびその製造法と応用」
笹井宏明, 滝澤忍, 松井嘉津也, 特開2006-28021
Original Paper
1.
Spiro Crown Ethers Bearing (S )-1,1'-Spirobiindanes
as Chiral Backbones
Yonezawa, K.; Patil, M. L.; Sasai, H.; Takizawa, S. Heterocycles 2005, 66, 639-644.
2.
Bifunctional Organocatalysts for
Enantioselective aza-Morita-Baylis-Hillman Reaction
Matsui, K.; Takizawa, S.; Sasai, H. J. Am. Chem. Soc. 2005, 127, 3680-3681. (DOI)
3.
Micelle-Derived Polymer Supports for
Enantioselective Catalysts
Takizawa, S.; Patil, M. L.; Yonezawa, F.; Marubayashi,
K.; Tanaka, H.; Kawai, T.; Sasai, H. Tetrahedron Lett. 2005, 46, 1193-1197. (DOI)
4.
Enantioselective Morita-Baylis-Hillman (MBH)
Reaction Promoted by a Heterobimetallic Complex with a Lewis Base
Matsui, K.; Takizawa, S.; Sasai, H. Tetrahedron Lett. 2005, 46, 1943-1946. (DOI)
5.
Effective Forms of Hydroxyapatite Disperse
Phase in Solvent-Free Epoxidation System
Iteya, K.; Sasaki, Y.; Itoh, S.; Ichihara, J. Phosphorus Res. Bull. 2005,
19, 142-146.
Patent
1.
「不斉合成用触媒の製造方法」
柴崎正勝, 笹井宏明, 田原義博, 特開2005-028363
2.
「エポキシ化合物の製造方法」
市原潤子, 佐々木 洋, 山口俊郎, 野須 勉, 特開2005-104902
Original Paper
1.
Dual Activation in a Homolytic Coupling
Reaction Promoted by an Enantioselective Dinuclear
Vanadium(IV) Catalyst
Somei, H.; Asano, Y.; Yoshida, T.; Takizawa, S.; Yamataka, H.; Sasai, H. Tetrahedron Lett. 2004, 45, 1841-1844. (DOI)
2.
Development of Novel Chiral Spiro Ligand
Bearing Oxazolines
Kato, T.; Marubayashi, K.; Takizawa, S.; Sasai, H. Tetrahedron: Asymmetry 2004,
15, 3693-3697. (DOI)
3.
Enantioselective Aldol-type Reaction Using
Diketene
Kawase, T.; Takizawa, S.; Jayaprakash, D.; Sasai, H. Synth. Commun. 2004, 34, 4487-4492. (DOI)
4.
Spiro Bis(isoxazole) as a New Chiral Ligand
Wakita, K.; Arai, M. A.; Kato, T.; Shinohara, T.; Sasai, H. Heterocycles 2004,
62, 831-838.
5.
Tetrabutylammonium Phosphomolybdate on
Fluorapatite: an Efficient Solid Catalyst for Solvent-free Selective Oxidation
of Sulfides
Sasaki, Y.; Ushimaru, K.; Iteya,
K.; Nakayama, H.; Yamaguchi, S.; Ichihara, J. Tetrahedron Lett. 2004, 45, 9513-9515. (DOI)
6.
Formation of Fluoridated Hydroxyapatite by
Competitive Attack of OH- and F- Ions onto a- or b-tricalcium
bis(orthophosphate)
Sakamoto, K.; Yamaguchi, S.; Ichihara, J.; Okazaki, M.; Tsunawaki,
Y.; Elliott, J. C. J. Ceram. Soc. Jpn. 2004,
112, 6-12. (DOI)
7.
The Use of Apatite as Green Disperse-phase
for Solvent-free Epoxidation
Sasaki, Y.; Ichihara, J.; Sakamoto, K.; Yamaguchi, S. Phosphorus Research Bull. 2004, 17, 215-218.
8.
Solvent-free Catalytic Oxidation System Using
Apatite Disperse Phase
Ichihara, J.; Iteya, K.; Kambara, A.; Yamaguchi, S.;
Sasaki, Y. Catalysts & Catalysis 2004, 46, 57-59.
9.
Asymmetric ligands bearing spiro skeleton and
their applications to enantioselective catalysis
Takizawa, S.; Jayaprakash, D.; Patil, M. L.; Muthiah, C.; Sasai, H. Materials Integration 2004,
17, 3-6.
10. Development
of novel immobilization methods for multifunctionl
asymmetric catalysts
Takizawa, S.; Sasai, H. 生産と技術 2004, 56, 43.
11. Trend in
the development of novel chiral ionic liquids
Patil, M. L.; Takizawa, S.; Sasai, H. Chemical Industry 2004, 55, 877.
12. スピロビスイソオキサゾリン配位子(SPRIXs)の創製と触媒的不斉合成ヘの応用
荒井緑, 篠原俊夫, 荒井孝義, 笹井宏明 有機合成化学協会誌 2004, 62, 59.
Original Paper
1.
Design and Synthesis of Novel Spiro
Pyridinium and Quinolinium Salts
Patil, M. L.; Takizawa, S.; Sasai, H. Heterocycles 2003, 61, 581.
2.
Metal-bridged Polymers as Insoluble
Multicomponent Asymmetric Catalysts with High Enantiocontrol: An Approach for
the Immobilization of Catalysts without Using any Support
Takizawa, S.; Somei, H.; Jayaprakash, D.; Sasai, H. Angew. Chem. Int. Ed. 2003,
42, 5711. (DOI)
3.
Monolayer-protected Au Cluster
(MPC)-supported Ti-BINOLate Complex
Marubayashi, K.; Takizawa, S.; Kawakusu, T.; Arai,
T.; Sasai, H. Org. Lett. 2003, 5, 4409. (DOI)
4.
Synthesis of Novel Chiral Spiro Bis(pyrazole)
Ligands
Takizawa, S.; Honda, Y.; Arai, M. A.; Kato, T.; Sasai, H. Heterocycles 2003,
60, 2551.
5.
Polymer Supported BisBINOL
Ligands for the Immobilization of Multicomponent Asymmetric Catalysts
Sekiguti, T.; Iizuka, Y.; Takizawa, S.; Jayaprakash,
D.; Arai, T.; Sasai, H. Org. Lett. 2003, 5, 2647. (DOI)
6.
Enantioselective Synthesis of a-Methylene-g-butyrolactones Using
Chiral Pd(II)-SPRIX Catalyst
Muthiah, C.; Arai, M. A.; Shinohara, T.; Arai, T.; Takizawa, S.; Sasai, H. Tetrahedron Lett. 2003,
44, 5201. (DOI)
7.
Enantioselective Epoxidation of a,b-Unsaturated Ketons
Using Polymer-supported Lanthanoid-BINOL Complexes
Jayaprakash, D.; Kobayashi, Y.; Watanabe, S.; Arai, T.; Sasai, H. Tetrahedron: Asymmetry 2003,
14, 1587. (DOI)
8.
Catalytic Asymmetric Epoxidation of a,b-Unsaturated Ketons
Using Polymeric BINOL
Jayaprakash, D.; Kobayashi, Y.; Arai, T.; Hu, Q.-S.; Zheng, X.-F.; Pu, L.;
Sasai, H. J. Mol. Catal. A:
Chem. 2003, 196, 145. (DOI)
9.
"Catalyst Analogue": A Concept for
Constructing Multicomponent Asymmetric Catalysts (MAC) Using a Polymer Support
Arai, T.; Sekiguti, T.; Otsuki, K.; Takizawa, S.;
Sasai, H. Angew. Chem. Int. Ed. 2003, 42, 2144. (DOI)
10. Synthesis
of New Chiral Bis(isoxazoline) Ligands Containing
Spiro[5.5]undecane Skeleton
Arai, M. A.; Kuraishi, M.; Arai, T.; Sasai, H. Chirality 2003,
15, 101. (DOI)
11. The First
Enantioselective Intramolecular Amino-carbonylation of Alkynes Promoted by
Pd(II)-Spiro Bis(isoxazoline) Catalyst
Shinohara, T.; Arai, M. A.; Wakita, K.; Arai, T.; Sasai, H. Tetrahedron Lett. 2003,
44, 711. (DOI)
12. Synthesis
and Character of New Bis(isoxazoline) Ligands
Shinohara, T.; Wakita, K.; Arai, M. A.; Arai, T.; Sasai, H. Heterocycles 2003,
59, 587.
13. The
Catalytic Activities of Nanoclusters Dispersed on Apatite
Ichihara, J.; Iteya, K.; Kawaguchi, H.; Sasaki, Y.;
Nakayama, H.; Yamaguchi, S. J. Ceram. Process. Res. 2003, 4, 42.
14. Cetylpyridinium Dodecatungstate on
Fluorapatite: Efficient and Reusable Solid Catalyst for Solvent-Free
Epoxidation
Ichihara, J.; Kambara, A.; Iteya, K.; Sugimoto, E.;
Shinkawa, T.; Takaoka, A.; Yamaguchi, S.; Sasaki, Y. Green Chem. 2003,
5, 491.
15. New
Approach to Environmentally Benign Epoxidation: The Solid-Phase-System Using
Urea-H2O2 and Recyclable Dodecatungstate on Apatite
Ichihara, J.; Iteya, K.; Kambara, A.; Sasaki, Y. Catal. Today 2003, 87, 163.