2026
Original Paper
1. Design
and Synthesis of Sorafenib-Inspired Benzofuran Hybrids as VEGFR-2 Inhibitors:
Antiproliferative Evaluation, Mechanistic Insights, and Docking Studies in
Hepatocellular Carcinoma
S. Shehda, A. M. Almatary, M. Salem,
M. H. Aboutaleb, S. Takizawa, Y. M. A. A. Yasmine, M. El-Sayed, R. Elrayess, RSC Med. Chem.
2026, 17,
477-493 (DOI)
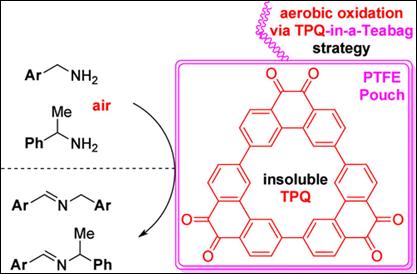
2. A Recyclable
Triangular Phenanthrenequinone (TPQ) Catalyst-in-a-Teabag System for Biomimetic
Aerobic Oxidation of Benzylamines
Oh, J. J.; Salem, H.; Takizawa,
S.; Kim, H. Y. Org. Lett. 2026,
28, 722-726. (DOI)
Book & Review
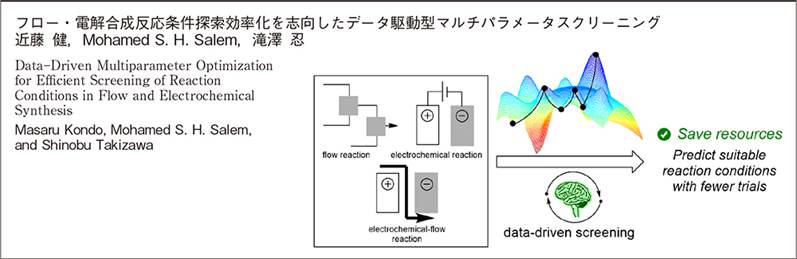
1. フロー・電解合成反応条件探索効率化を志向したデータ駆動型マルチパラメータスクリーニング
近藤 健, Mohamed S. H. Salem, 滝澤 忍, 有機合成化学協会誌, 2026, 84, 41-49 (DOI)
2025
Original Paper
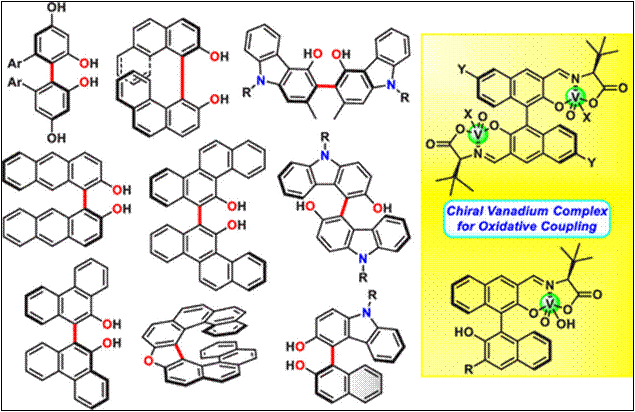
1. Enantioselective
hetero-coupling of 2-naphthylamines with 2-naphthol derivatives via
cooperative photo-activation and chiral vanadium(V) catalysis
D. Fan, M.
Karuppasamy, G. Kamble, K. Ando, D. Zhou, M. Salem, H. Sasai, S. Takizawa,
ACS Catal. 2025, 15, 18077 (DOI). <Selected as a Cover Picture>

2. Antimicrobial,
Structural, Optical, and Redox Profiling of 7H-Benzo[c]carbazol-10-ol
Derivatives: An Integrated Experimental and Computational Study
M. Salem,
M. E. l. Samak, Y. A. Aziz, M. Aboutaleb, S. Patil, T. Z. Aye, T. Ibrahim, S.
Takizawa, ACS Omega 2025, 10, 59183 (DOI). <Selected as a Cover Picture>

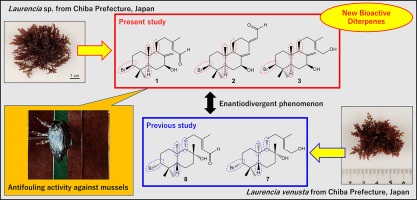
3. Chemical
structure and antifouling activity of yoshioaplysins
A–C isolated from red alga genus Laurencia
R. Fukada,
K. Nishikawa, M. Nagasaka, M. Kirihara, S. Takizawa, Y. Morimoto, K. Nimura, N.
Kikuchi, Y. Yamagishi, T. Ishii, T. Kamada, Fitoterapia
2025, 185,
106683 (DOI).
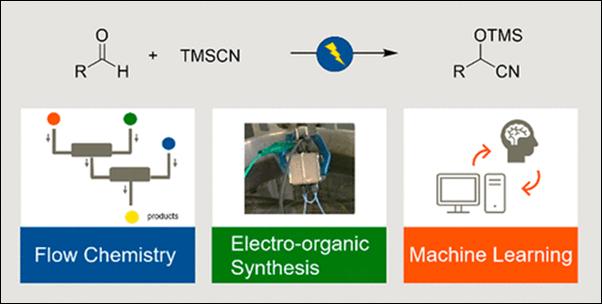
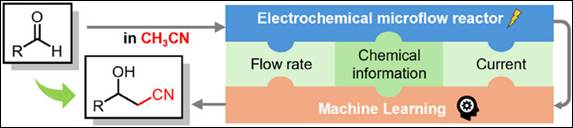
4. Cyanomethylation
of Aldehydes on an Electrochemical Microflow System and Utility of Machine
Learning-Assisted Examination of the Reaction Conditions
E.
Sato, A. Tani, T. Miyao, S. Kunimoto, S. Takizawa, K. Mitsudo,
S. Suga, Chem. Eur. J.
2025, 31, e202501257(DOI).
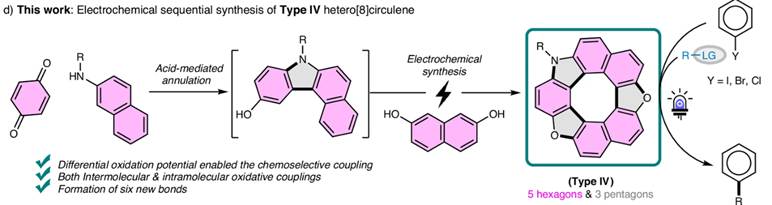
5. Electrochemical
Cascade Access to Hetero[8]circulenes: Potent Organophotocatalysts
for Diverse C–X Bond Formations
S.
Gabr, M. S. H. Salem, M. I. Khalid, R. Takahashi, Y. Nishimoto, M. Yasuda, S. Takizawa Nat. Commun. 2025, 16,
5682(DOI).
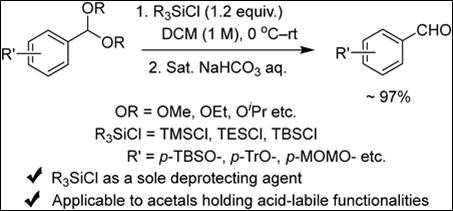
6. Unexpected
Surprise in the Reactions of Acetals and Trialkylsilyl
Chloride (R3SiCl): Efficient Deprotection of Aromatic Acyclic
Acetals
M.
Karuppasamy, I. U. Wedage, M. S. H. Salem, K. Morimoto, S. Takizawa, H. Fujioka, Chem. Pharm. Bull. 2025, 73, 396-400 (DOI).
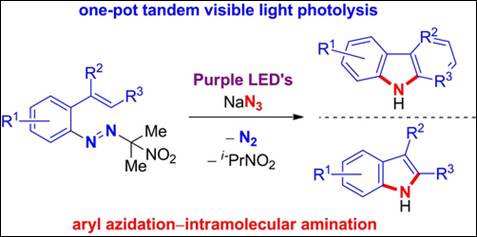
7.
Tandem Photocatalyst-Free Visible Light Aryl
Azidation-Intramolecular C-H Amination: One-Pot Access to Carbazoles and
Indoles from Areneazo-2-(2-nitro)propanes
D. V. Patil, R. Patel, S. Takizawa,
H. Y. Kim, K. Oh, Adv. Synth. Catal. 2025, 367,
e202401333 (DOI).
Book &
Review
1.
Remodelling Molecular Frameworks via
Atom-Level Surgery: Recent Advances in Skeletal Editing of (Hetero)Cycles
R. Sharma, M. Arisawa,
S. Takizawa, M. S. H. Salem, Org. Chem. Front. 2025, 12, 1633-1670 (DOI).
2. Few
Steps, Big Impact: Recent Advances in the Streamlined Synthesis of Multiply
Chiral Heterohelicenes with Tunable Optoelectronic Properties
M.
S. H. Salem, S. Takizawa, Chemistry-Methods 2025, 5, e202500018 (DOI). <Selected as a Cover Picture>

3.
フロー合成におけるベイズ最適化による有機分子触媒反応のマルチパラメータスクリーニング
近藤健, M. S. H. Salem, 滝澤忍, 月間ファインケミカル, 【特集】有機分子触媒:加速する触媒化学の現在地(2), シーエムシー出版 2025年9月15日出版
4.
機械学習を活用する精密有機合成反応条件の迅速最適化
滝澤 忍,M.
S. H. Salem,,生産と技術 2025, 77(2), 93-96.
2024
Original
Paper
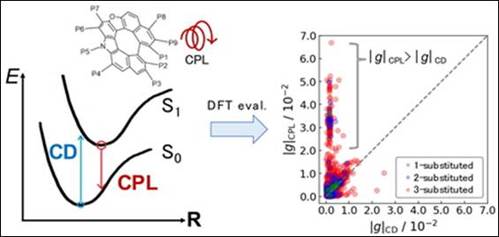
1.
Theoretical Study on the Relationship between Circular
Dichroism and Circularly Polarized Luminescence in Oxaza[7]dehydrohelicene Derivatives
M. FUJIWARA, M. FUJINAMI, M. S. H.
SALEM, S. TAKIZAWA, H. NAKAI, J. Comput. Chem. Jpn. 2024, 23, 37-39 (DOI).
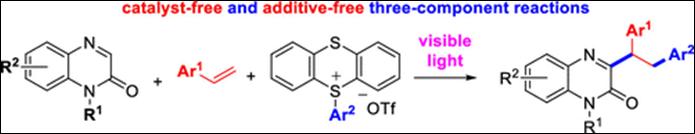
2.
Visible Light-Induced Radical Cascade Functionalization of
Quinoxalin-2(1H)-ones: Three-Component 1,2-Di(hetero)arylation Approach with Styrenes and Thianthrenium Salts
Sau, S.; Takizawa, S.; Kim, H. Y.;
Oh, K. Org.
Lett. 2024,
26, 8821-8826 (DOI).
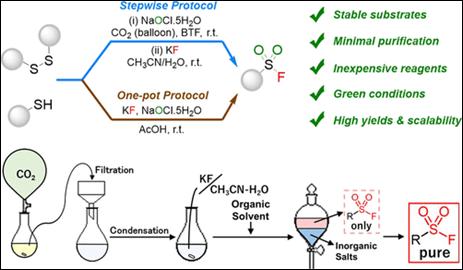
3.
Green and Efficient Protocols for the Synthesis of Sulfonyl
Fluorides Using Potassium Fluoride as the Sole Fluorine Source
Yamahara, S.; Salem, M. S. H.;
Kawai , T.; Watanabe, M.; Sakamoto, Y.; Okada, T.; Kimura, Y.; Takizawa, S.;
Kirihara, M. ACS
Sustainable Chem. Eng. 2024, 12, 12135-12142 (DOI). <Selected as a Cover Picture>

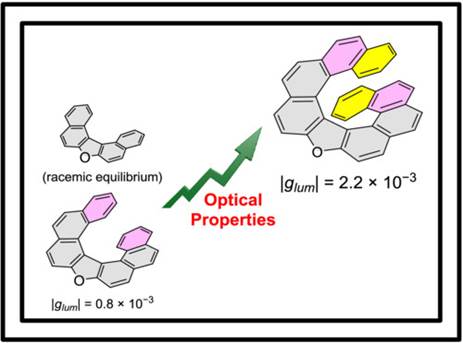
4.
Impact of helical elongation of symmetric oxa[n]helicenes
on their structural, photophysical, and chiroptical characteristics
Salem, M. S. H.; Sharma, R.; Suzuki,
S.; Imai, Y.; Arisawa, M.; Takizawa, S. Chirality 2024, 36, e23673 (DOI).
5.
Light-induced autoxidation of aldehydes to peracids and
carboxylic acids
Salem, M. S. H.; Dubois, C.;
Takamura, Y.; Kitajima, A.; Kawai, T.; Takizawa, S.; Kirihara, M. Green Chem. 2024, 26, 375 (DOI).
6.
Selective Recognition between Aromatics and Aliphatics by Cage-Shaped Borates Supported by Machine
Learning Approach
Tsutsui, Y.; Yanaka, I.; Takeda, K.;
Kondo, M.; Takizawa, S.; Kojima, R.; Konishi, A.; Yasuda, M. Org. Biomol.
Chem. 2024, 22,
4283-4291 (DOI). <Selected as a Cover Picture>

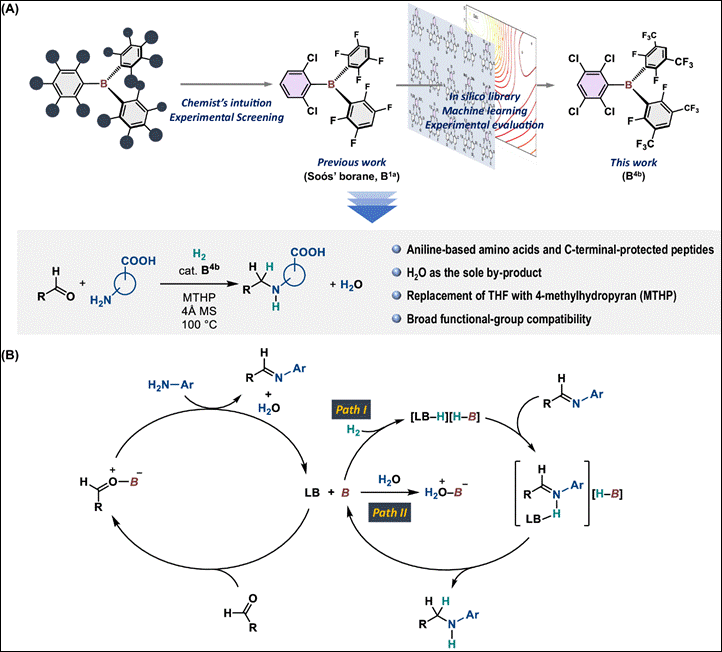
7.
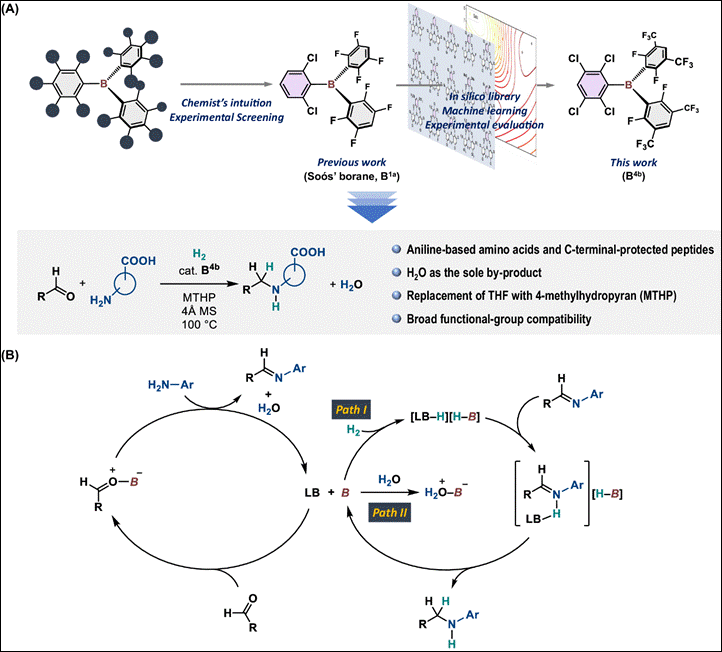
In-Silico-Assisted Derivatization of Triarylboranes
for the Catalytic Reductive Functionalization of Aniline-Derived Amino Acids
and Peptides with H2
Hisata, Y.; Washio, T.; Takizawa, S.;
Ogoshi, S.; Hoshimoto, Y. Nat. Commun. 2024,
15, 3708 (DOI).
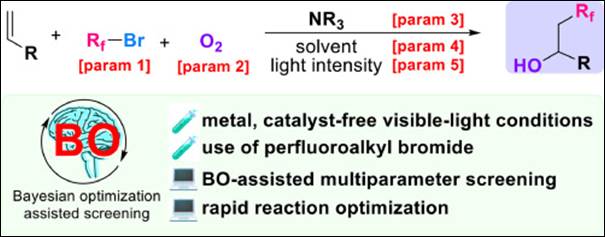
8.
Bayesian optimization assisted screening conditions for
visible light-induced hydroxy-perfluoroalkylation
Tagami, K.; Kondo, M.; Takizawa, S.;
Mase, N.; Yajima, T. J. Fluor. Chem. 2024, 276, 110294 (DOI).

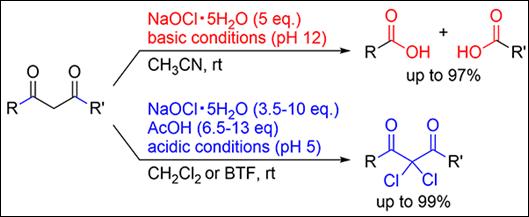
9.
Product Selectivity Control under Acidic and Basic Conditions
on Oxidative Transformation of 1,3-Dicarbonyls Using Sodium Hypochlorite
Pentahydrate
Kirihara, M.; Sakamoto, Y.; Tanaka,
T.; Kawai, T.; Okada, T.; Kimura, Y.; Takizawa, S. Synthesis 2024, 56, 1873-1880 (DOI)
Book &
Review
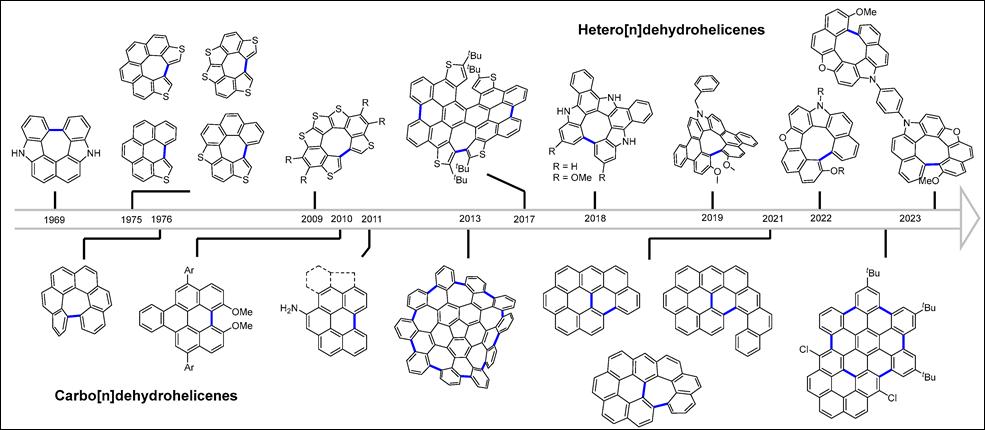
1.
Synthesis and Structural and Optical Behavior of Dehydrohelicene-Containing Polycyclic Compounds
Khalid, Md. I.; Salem, M. S. H.;
Takizawa, S. Molecules 2024, 29,
296 (DOI).
2.
Organocatalyzed Enantioselective Synthesis of Spirooxindole Scaffolds
M. S. H. Salem, A. Sabri, M. Sasi, D.
Fan, S. Takizawa (K. Deshmukh, T. A. Nguyen, G. Patel, V. R. Shah Eds), Spirooxindole: Chemistry, synthesis,
characterization and biological significance, Elsevier, 2024年6月12日出版
3.
Atropisomerism in asymmetric organic
synthesis: Challenges and applications
M. S. H. Salem, S. Takizawa Eds,
Wiley-VCH Verlag GmbH & Co. KGaA, 2024年10月7日出版
4.
機械学習を活用したフロー精密有機合成の可変パラメータ最適化
滝澤忍,
ファインケミカル, 医薬品の連続生産プロセス, 技術情報協会, 2024年11月29日出版
2023
Original
Paper
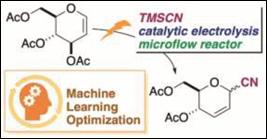
1.
Electrochemical Carbon-Ferrier Rearrangement Using a
Microflow Reactor and Machine Learning-assisted Exploration of Suitable
Conditions
Sato, E.; Tachiwaki,
G.; Fujii, M.; Mitsudo, K.; Washio, T.; Takizawa, S.;
Suga, S. Org. Process Res. Dev.
2023, in press (DOI).
2.
Data-driven Electrochemical One-pot Synthesis of Double
Hetero[7]dehydrohelicene
Salem, M. S. H.; Sharma, R.; Khalid,
Md. I.; Sasi, M.; Amasaki, R.; Imai, Y.; Arisawa, M.;
Takizawa S.
Electrochemistry
2023, 91, 112015 (DOI).
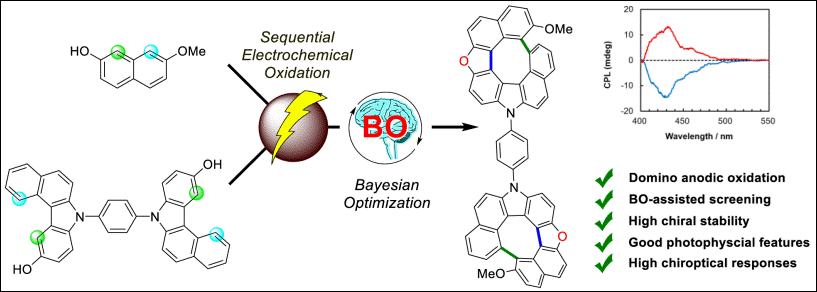
3. Antifouling Brominated Diterpenoids from Japanese Marine Red Alga
Laurencia venusta Yamada
Fukada, R.; Yamagishi, Y.; Nagasaka, M.; Osada, D.; Nimura, K.;
Oshima, I.; Tsujimoto, K.; Kirihara, M.; Takizawa, S.; Kikuchi, N.; Ishii, T.; Kamada, T. Chem. Biodiversity 2023, 20,
e202300888 (DOI).
4. Light-controlled pKa Value of Chiral
Brønsted Acid Catalysts in Enantioselective Aza-Friedel–Crafts Reaction
Krishnan,
C. G.; Kondo, M.; Yasuda, Y.; Fan, D.; Nakamura, K.; Wakabayashi, Y.; Sasai,
H.; Takizawa, S. Chem. Commun. 2023, 59, 9956 (DOI). <Selected as a Cover Picture>
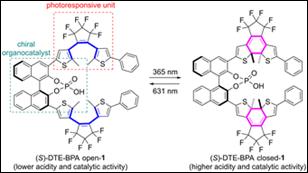

5.
Bayesian Optimization-assisted Screening to Identify Improved
Reaction Conditions for Spiro-dithiolane Synthesis
Kondo, M.; Wathsala, H. D. P.;
Ishikawa, K.; Yamashita, D.; Miyazaki, T.; Ohno, Y.; Sasai, H.; Washio, T.;
Takizawa, S. Molecules, 2023,
28, 5180 (DOI).
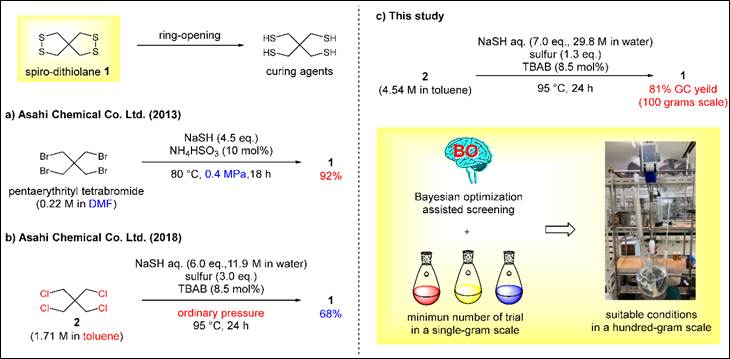
6.
Electrochemical Synthesis of Hetero[7]helicenes Containing
Pyrrole and Furan Rings via an Oxidative Heterocoupling
and Dehydrative Cyclization Sequence
Salem, M. S. H.; Khalid, Md. I.;
Sako, M.; Higashida, K.; Lacroix, C.; Kondo, M.; Takishima,
R.; Taniguchi, T.; Miura, M.;
Vo-Thanh, G.; Sasai, H.; Takizawa, S. Adv. Synth. Catal. 2023, 365,
373 (DOI).<Top Viewed
Article>
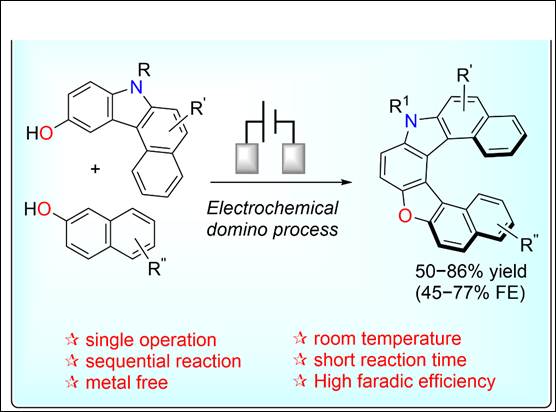
7. Two-pot synthesis of
unsymmetrical hetero[7]helicenes with intriguing optical properties
Salem, M. S. H.; Khalid,
Md. I.; Sasai, H.; Takizawa, S. Tetrahedron 2023, 133, 133266 (DOI).
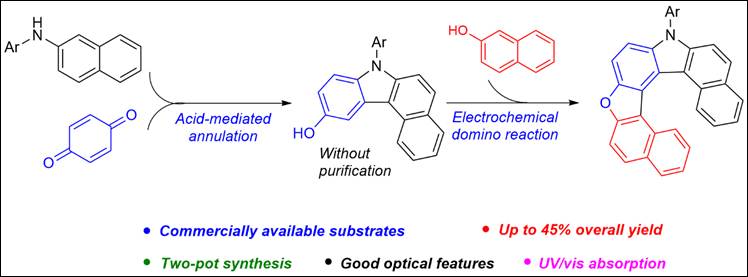
8.
Metal-Free Aerobic C–N Bond Formation of Styrene and Arylamines
via Photoactivated Electron Donor-Acceptor Complexation
Fan, D.; Sabri, A.; Sasai, H.;
Takizawa, S. Molecules 2023, 28, 356 (DOI).
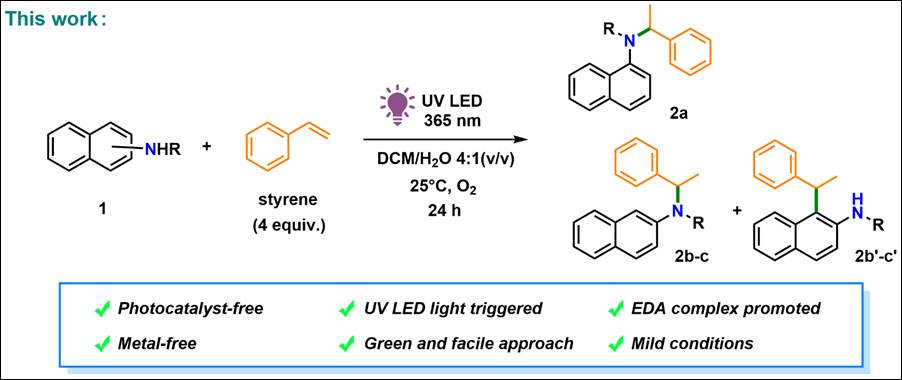
Book &
Review
1.
Photoswitchable Chiral Organocatalysts: Photocontrol of Enantioselective Reactions
Kondo, M.; Nakamura, K.; Krishnan,
C.G.; Sasai, H.; Takizawa, S. Chem. Rec. 2023, 23, e202300040 (DOI).
2.
Azobenzene─based Chiral Photoswitchable Catalysts
Kondo, M.; Nakamura, K.;
Sasai, H.; Takizawa, S. J. Synth. Org. Chem. Jpn. 2023, 81, 817 (DOI).
3.
ベイズ最適化による電解・フロー精密有機合成反応条件の探索と効率化
近藤健,
滝澤忍, 実験の自動化・自律化によるR&Dの効率化と運用方法, 技術情報協会, 2023年12月27日出版
Original
Paper
1.
Bayesian optimization with constraint on passed charge for multiparameter
screening of electrochemical reductive carboxylation in a flow microreactor
Naito, Y.; Kondo, M.; Nakamura, Y.; Shida, N.; Ishikawa, K.;
Washio, T.; Takizawa, S.; Atobe, M. Chem. Commun. 2022, 58, 3893 (DOI) (Selected as Inside Front Cover).
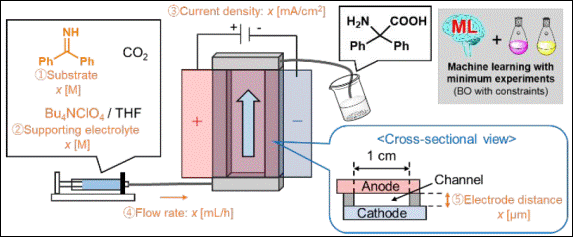

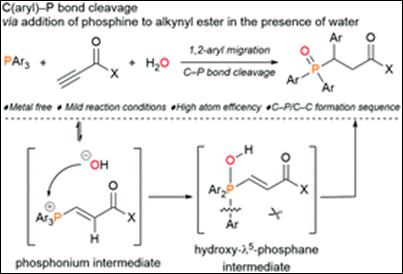
2.
Metal-free C(aryl)–P bond cleavage: experimental and computational
studies of the Michael addition/aryl migration of triarylphosphines
to alkenyl esters
Sako, M.; Kanomata,
K.; Mohamed, S. H. S.; Furukawa, T.; Sasai H.; Takizawa, S. Org. Chem. Front. 2022, 9, 2187 (DOI).
3.
Photoswitchable chiral cation-binding
catalyst: Photocontrol of catalytic activity on enantioselective aminal synthesis
Krishnan, C.; Kondo, M.;
Nakamura, K.; Sasai, H.; Takizawa, S. Org. Lett. 2022, 24, 2670 (DOI) (Selected as Front Cover).

4. DAST-mediated ring-opening of cyclopropyl silyl ethers in nitriles: Facile synthesis of allylic amides via Ritter-type process
Kirihara, M.; Nakamura, R.; Nakakura,
K.; Tujimoto, K.; Mohamed S. H. S.; Suzuki, T.;
Takizawa, S. Org. Biomol. Chem. 2022, 20, 6558 (DOI).

5. New anionic cobalt(III) complexes enable
enantioselective synthesis of spiro-fused oxazoline and iodoacetal derivatives
Salem, M.
S. H. and Takizawa, S. Front.
Chem. 2022, 10:1034291 (DOI).
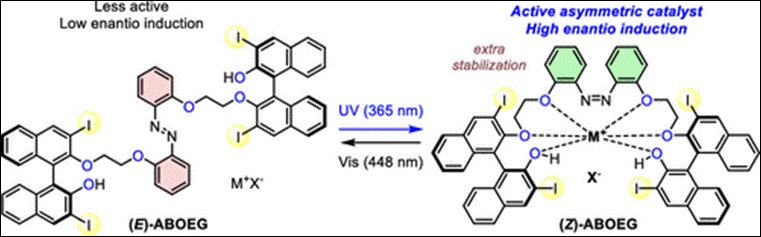
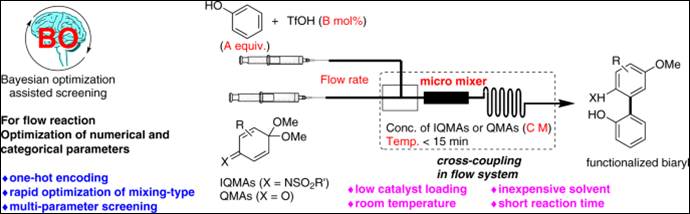
6. Bayesian
optimization-driven parallel-screening on multi-parameters of micromixer-type
and organocatalytic conditions in the flow biaryl
synthesis
Kondo, M.; Wathsala, H. D. P.; Salem, M. S. H.; Ishikawa, H.; Hara, S.; Takaai, T.; Washio, T.; Sasai, H.; Takizawa, S. Commun. Chem. 2022, 5,
148. (DOI)
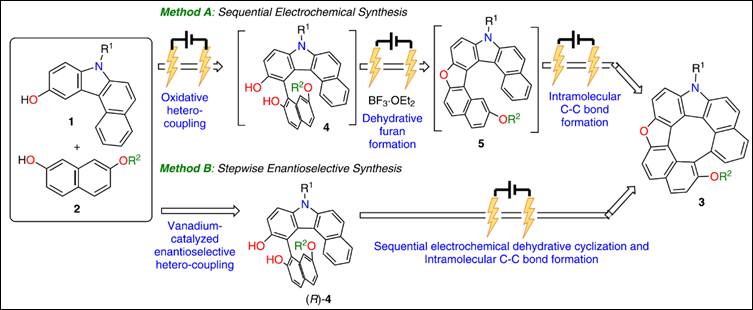
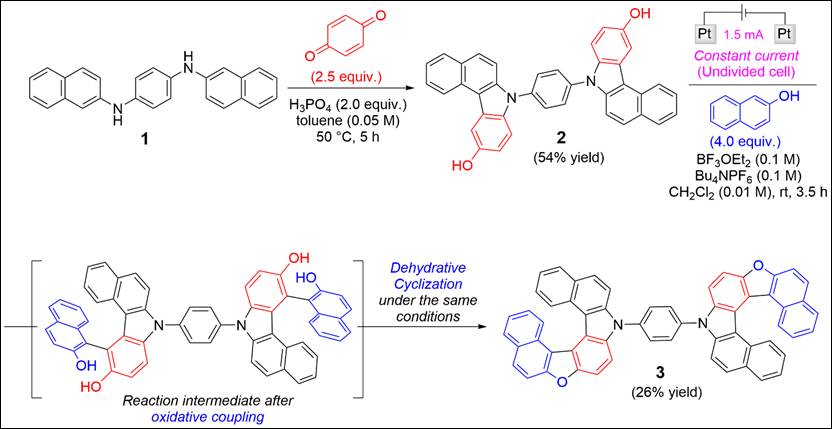
7. Electrochemical synthesis of heterodehydro[7]helicenes
Khalid, Md. I.; Salem, M. S. H.; Sako, M.; Kondo, M.; Sasai, H.; Takizawa, S. Commun. Chem. 2022, 5, 166 (DOI).
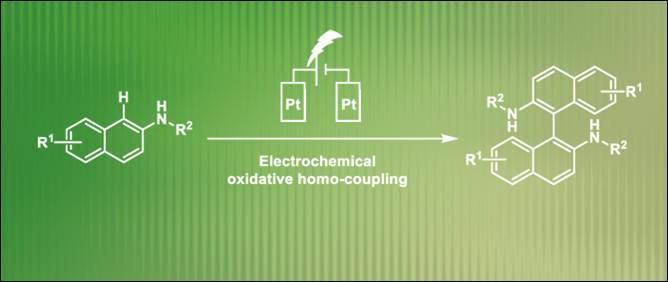
8.
Electrochemical
Synthesis of 1,1’-Binaphthalene-2,2’-diamines via Transition-Metal-Free
Oxidative Homocoupling
Fan, D.; Khalid, Md. I.; Kamble, G. T.; Sasai, H.; Takizawa, S. Sustain. Chem. 2022, 3, 551 (DOI) (Highlighted
on the Main Page of the Journal).
Hiro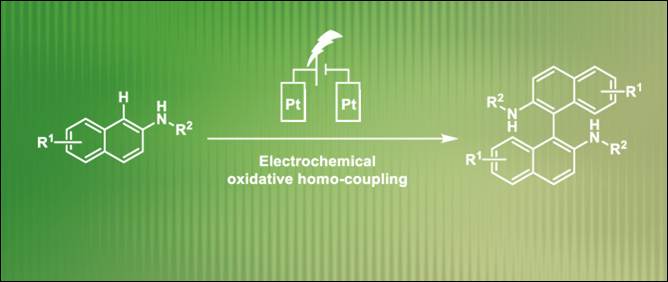
9.
Two-Step Synthesis, Structure, and Optical
Features of a Double Hetero[7]helicene
Salem, M.
S. H.; Sabri, A.; Khalid, I.; Sasai, H.; Takizawa, S. Molecules 2022, 27, 9068 (DOI).
Book
& Review
1.
Atroposelective synthesis of C–C
axially chiral compounds via mono- and dinuclear
vanadium catalysis
Kumar,
A.; Sasai, H.; Takizawa, S. Acc. Chem. Res.
2022, 55, 2949 (DOI).
2021
Original
Paper
1.
Preparation of Optically Pure Dinuclear Cobalt(III) Complex with Λ–Configuration as a Dianionic Chiral Catalyst
Salem, M. S. H.;
Kumar, A.; Sako, M.; Abe, T.; Takizawa, S.; Sasai, H. Heterocycles, 2021, 103, 225 (DOI).

2.
Photoswitchable
Chiral Phase Transfer Catalyst
Kondo, M.; Nakamura, K.; Krishnan,
C.; Takizawa, S.; Abe, T.; Sasai, H. ACS
Catalysis, 2021, 11, 1863(DOI).
<Highlighted
in Synfacts 2021, 17, 442. (DOI)>
3.
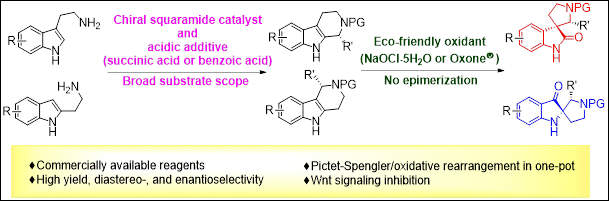
Practical Stereoselective Synthesis of C3‐Spirooxindole‐ and C2‐Spiropseudoindoxyl‐Pyrrolidines via Organocatalyzed Pictet‐Spengler
Reaction/Oxidative Rearrangement Sequence
Kondo,
M.; Matsuyama, N.; Aye, T. Z.; Mattan, I.; Sato, T.; Makita, Y.; Ishibashi, M.;
Arai, M.; Takizawa, S.; Sasai, H. Adv. Synth. Catal., 2021, 363, 2648(DOI).
4.
Chemo- and Enantioselective
Hetero-coupling of Hydroxycarbazoles Catalyzed by a Chiral Vanadium(v) complex
Sako, M.; Higashida, K.; Kamble, G.
T.; Kaut, K.; Kumar, A.; Hirose, Y.; Zhou, D.; Suzuki, T.; Rueping,
M.; Maegawa, T.; Takizawa, S.; Sasai, H. Org. Chem. Front., 2021, 8, 4878 (DOI).
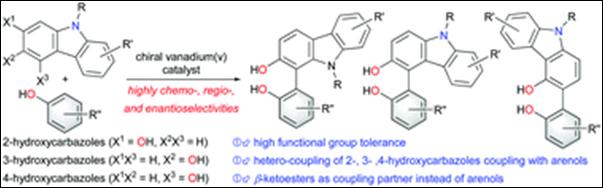
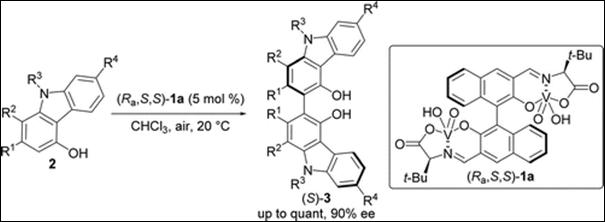
5.
Chiral Vanadium(v)-catalyzed Oxidative Coupling of
4-Hydroxycarbazoles
Kamble, G.; Salem, M.; Abe, T.;
Park, H.; Sako, M.; Takizawa, S.; Sasai, H. Chem. Lett., 2021,
50, 1755-1757 (DOI).
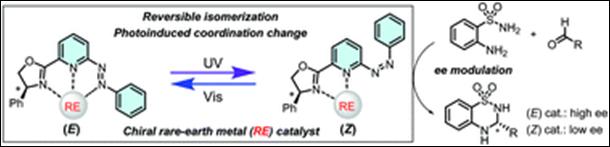
6.
Azopyridine-based
Chiral Oxazolines with Rare-earth Metals for Photoswitchable Catalysis
Chem. Commun., 2021,
57, 7414 (DOI).
7.
Energy-, Time-, and Labor-saving
Synthesis of α-Ketiminophosphonates:
Machine-learning-assisted Simultaneous Multiparameter Screening for
Electrochemical Oxidation

8. Application of an Electrochemical Microflow Reactor for Cyanosilylation: Machine Learning-assisted Exploration of
Suitable Reaction Conditions for Semi-large-scale Synthesis
, E.; , S. J. Org. Chem., 2021,86, 16035-16044 (DOI).
9.
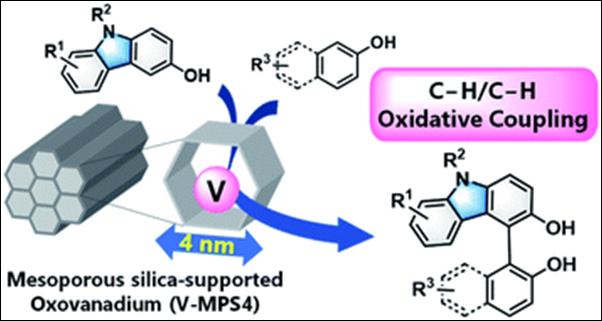
Chemo- and Regioselective Cross-dehydrogenative Coupling
Reaction of 3-Hydroxycarbazoles with Arenols Catalyzed by a Mesoporous
Silica-supported Oxovanadium
Kasama, K.; Kanomata, K.; Hinami, Y.; Mizuno, K.; Uetake, Y.; Amaya, T.; Sako, M.; Takizawa, S.; Sasai, H.; Akai, S. RSC Adv. 2021, 11, 35342-35350 (DOI).
Book
& Review
1.
不斉1,4-付加反応, 不斉森田–Baylis–Hilman反応
笹井宏明, 滝澤忍 (檜山為次郎, 野崎京子, 中尾佳亮, 中野幸司
編集), In 有機合成のための新触媒反応101, 東京化学同人, 2021年11月8日出版
2020
Original Paper
1.
Vanadium(V) Complex-catalyzed One-pot
Synthesis of Phenanthridines via a
Pictet-Spengler-Dehydrogenative Aromatization Sequence
Sako, M.; Losa, R.; Takiishi, T.;
Vo-Thanh, G.; Takizawa, S.; Sasai, H. Catalysts 2020, 10, 860(DOI)
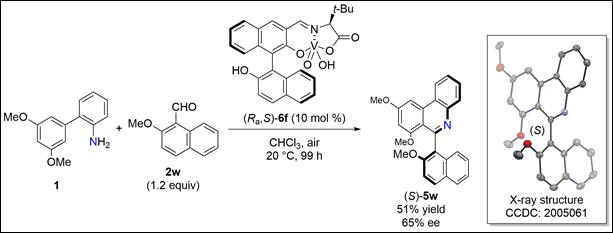
2. Catalytic
and Enantioselective oxa-Piancatelli Reaction using
Chiral Vanadium Complex
Schober,
L.; Sako, M.; Takizawa, S.; Gröger, H.; Sasai, H. Chem. Commun. 2020, 56, 10151-10154(DOI)

3. Synthesis
of Allylamine Derivatives via
Intermolecular Aza-Wacker-Type Reaction Promoted by Palladium–SPRIX Catalyst
Sen,
A.; Zhu, L.; Takizawa, S.; Takenaka, K.; Sasai, H. Adv. Synth. Catal. 2020, 362, 3558-3563(DOI)

4.
Exploration of Flow
Reaction Conditions Using Machine-learning for Enantioselective Organocatalyzed
Rauhut-Currier and [3+2] Annulation Sequence
Kondo, M.; Wathsala, H. D. P.; Sako, M.; Hanatani, Y.; Ishikawa, K.; Hara, S.; Takaai, T.; Washio, T.; Takizawa, S.; Sasai, H. Chem.
Commun. 2020, 56, 1259-1262. (DOI)
<Highlighted in Synfacts 2020, 16, 366. (DOI)>
<本研究成果はプレスリリースされました>
EurekAlert!(https://eurekalert.org/pub_releases/2020-02/ou-trs021820.php)
AlphaGalileo(https://www.alphagalileo.org/Item-Display/ItemId/189379)
5.
Enantioselective
One-pot Synthesis of 3-Azabicyclo[3.1.0]hexanes via Allylic Substitution and
Oxidative Cyclization
Chaki, B. M.; Takenaka, K.; Zhu, L.; Tsujihara, T.; Takizawa, S.; Sasai, H. Adv.
Synth. Catal. 2020, 362(7), 1537-1547. (DOI)
<Selected as a Very Important Publication (VIP)>
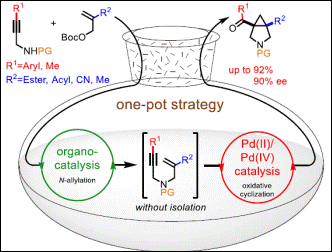
<Highlighted in Synfacts 2020, 16, 781. (DOI)>
Book & Review
1.
Organocatalytic Synthesis of Highly Functionalized
Heterocycles by Enantioselective aza-Morita–Baylis–Hillman-type Domino Reactions
Takizawa, S. Chem. Pharm. Bull. 2020, 68, 299-315.📚
<2019年度日本薬学会学術振興賞受賞記念総説>
2. Chiral vanadium complex-catalyzed oxidative coupling of
arenols
Sako, M.; Takizawa, S.; Sasai, H.
Tetrahedron 2020, 76, 131645. (DOI)
3.
Vanadium-catalyzed enantioselective C–C bond-forming reactions
M. Sako, S. Takizawa, H Sasai (M.
Sutradhar, J. A. L. da Silva, A. J. L. Pombeiro,
Eds), In Vanadium Catalysis, RSC Publishing, 2020年11月12日出版(Chapter 18)
2019
Original
Paper
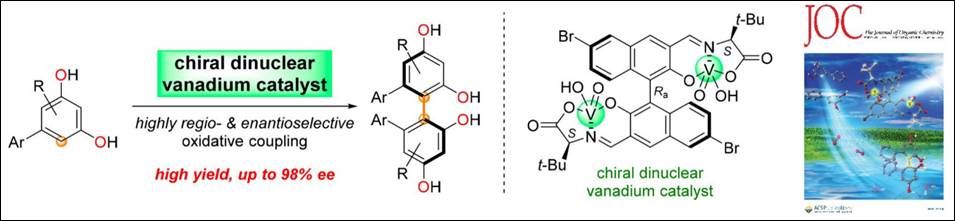
1.
Chiral Dinuclear
Vanadium Complex-mediated Oxidative Coupling of Resorcinols
Sako, M.; Aoki, T.; Zumbrägel, N.; Schober, L.;
Gröger, H.; Takizawa, S.; Sasai, H. J. Org. Chem. 2019, 84(3), 1580-1587. (DOI)
<Selected as a Cover Picture>
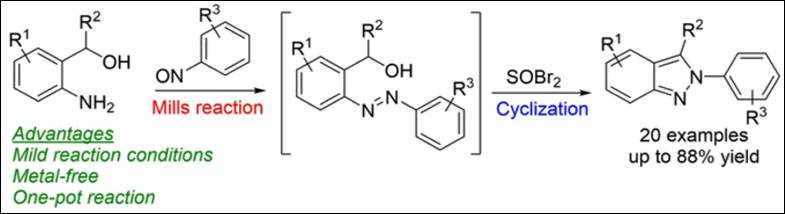
2.
Room-Temperature,
Metal-Free and One-Pot Preparation of 2H-Indazoles
via Mills Reaction and Cyclization
Sequence
Kondo, M.; Takizawa, S.; Jiang, Y.; Sasai, H. Chem. Eur. J. 2019, 25(42), 9866-9869. (DOI)
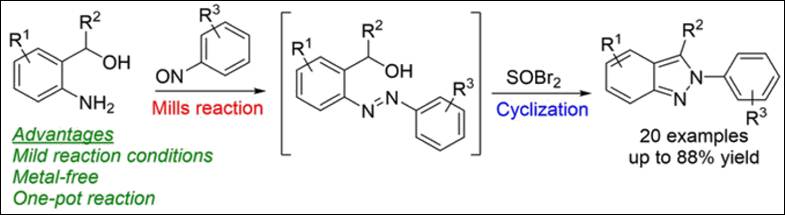
Book &
Review
1.
キラルバナジウム触媒によるヘリセン様化合物の簡便合成
佐古 真,滝澤 忍,笹井 宏明,生産と技術 2019, 71(2), 77-80.
Original
Paper
1. Asymmetric
Oxidative Coupling of Hydroxycarbazoles: Facile
Synthesis of (+)-Bi-2-hydroxy-3-methylcarbazole
Sako, M.; Sugizaki, A.; Takizawa, S. Bioorg. Med.
Chem. Lett. 2018, 28,
2751-2753. (DOI)
(Dedicated to Professor Dr. Dale L. Boger on the occasion of his 65th
birthday.)
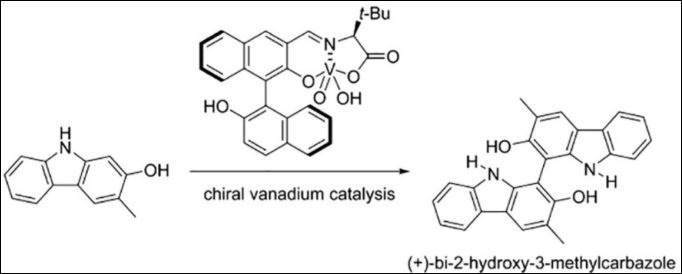
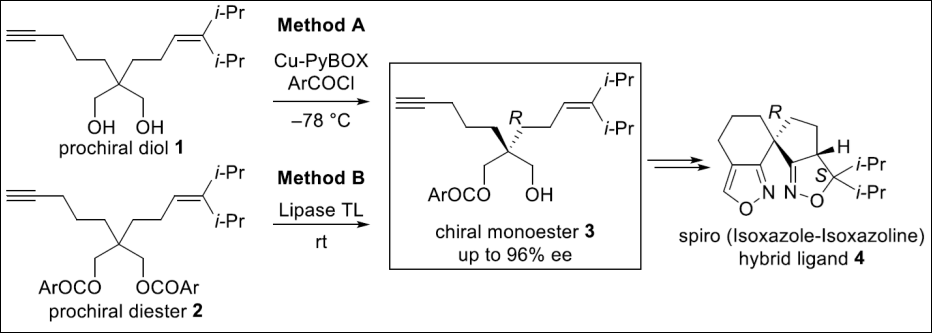
2. Enantioselective
Synthesis of Spiro (isoxazole-isoxazoline) Hybrid
Ligand
Chaki, B. M.; Wakita, K.; Takizawa, S.; Takenaka, K.; Sasai, H. Heterocycles 2018, 97, 493–505. (DOI)
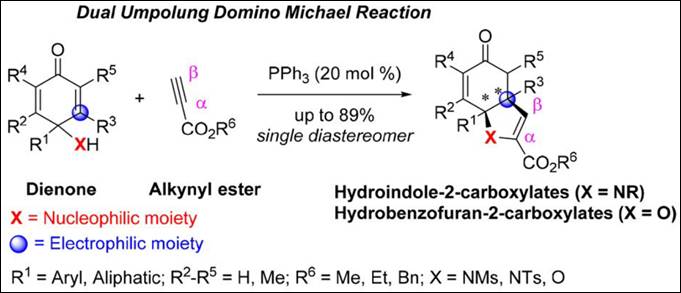
3. Phosphine-Catalyzed
Dual Umpolung Domino Michael Reaction: Facile Synthesis of Hydroindole-
and Hydrobenzofuran-2-Carboxylates
Kishi, K.; Takizawa, S.; Sasai, H. ACS Catal. 2018,
8, 5228-5232. (DOI)
4. Vanadium-Catalyzed
Dehydrogenation of N‑Heterocycles in
Water
Zumbrägel, N.; Sako, M.; Takizawa, S.; Sasai, H.;
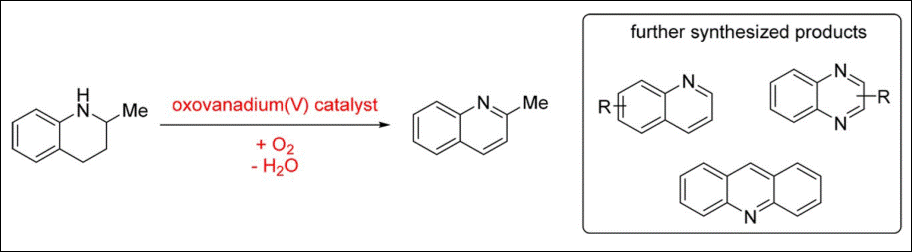
Gröger, H. Org. Lett. 2018, 20, 4723-4727. (DOI)
Book &
Review
1. “多機能有機分子不斉触媒を用いる環境調和型ドミノ反応の開発”
「有機分子触媒の開発と工業利用」
笹井宏明、滝澤忍,シーエムシー出版 (2018) 220-232.📚
2. “キラルバナジウム触媒を用いるエナンチオ選択的酸化カップリング反応の開発と応用”
佐古真、滝澤忍、笹井宏明,有機合成化学協会誌,Vol.76,
No.9 (2018),874-884.📚
“Chiral Vanadium Complex-catalyzed
Enantioselective Oxidative Coupling Reactions”
Sako, M.; Takizawa, S.; Sasai, H. J. Synth. Org. Chem. Jpn.
2018, 76, 874–884. (DOI)
Original
Paper
1. Facile
Synthesis of Spirooxindoles via an Enantioselective
Organocatalyzed Sequential Reaction of Oxindoles with Ynone
Takizawa, S.;
Kishi, K.; Kusaba, M.; Bai, J.; Suzuki, T.; Sasai, H. Heterocycles 2017, 95(2), 761-767. (DOI)

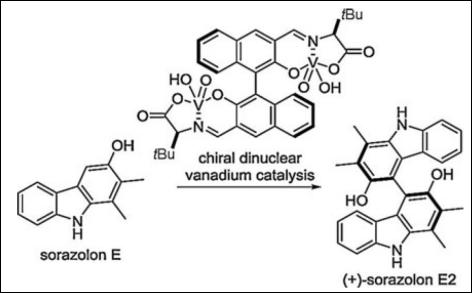
2. Short Syntheses of 4-Deoxycarbazomycin B, Sorazolon E, and (+)-Sorazolon E2
Sako, M.; Ichinose, K.; Takizawa, S.; Sasai, H. Chem. Asian J. 2017, 12(12),
1305–1308. (DOI)
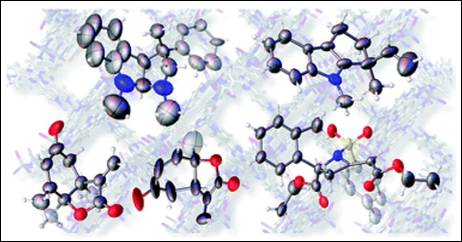
3. Determination
of the Absolute Configuration of Compounds Bearing Chiral Quaternary Carbon
Centers Using the Crystalline Sponge Method
Sairenji, S.; Kikuchi, T.; Abozeid, M. A.; Takizawa, S.; Sasai,
H.; Ando, Y.; Ohmatsu, K.; Ooi, T.; Fujita, M. Chem. Sci. 2017, 8(7), 5132–5136. (DOI)
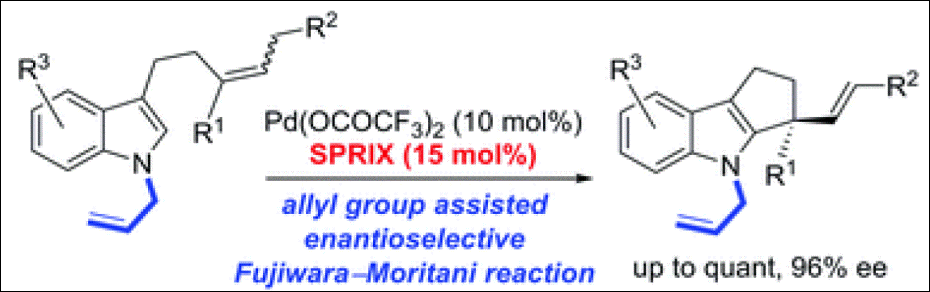
4. Enantioselective Synthesis
of Tetrahydrocyclopenta[b]indole Bearing a Chiral Quaternary Carbon Center via
Pd(II)-SPRIX-Catalyzed C–H Activation
Abozeid, M. A.; Sairenji, S.; Takizawa, S.; Fujita, M.;
Sasai, H. Chem. Commun. 2017,
53, 6887-6890. (DOI)
5. Multifunctional Catalysis:
Stereoselective Construction of α-Methylidene-γ-Lactams
via Amidation/Rauhut–Currier
Sequence
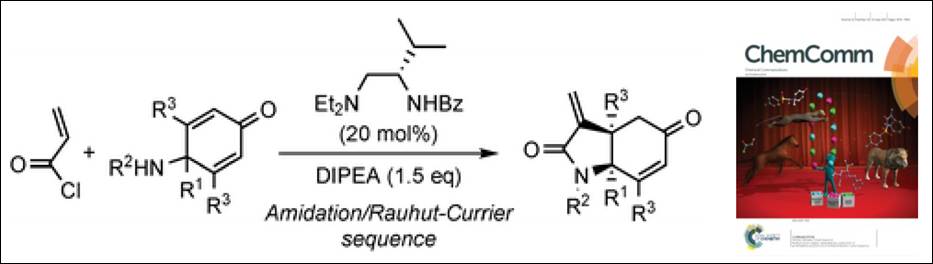
Kishi, K.; Arteaga, F. A.; Takizawa, S.; Sasai, H. Chem. Commun. 2017, 53. 7724-7727. (DOI)
<Selected as an
Inside Front Cover>
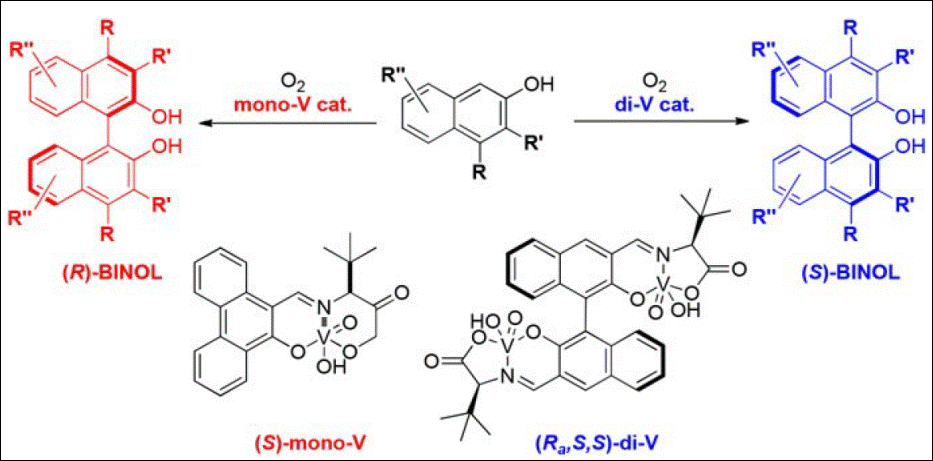
6. Reversal of
Enantioselectivity Approach to BINOLs via Single and Dual 2‑Naphthol Activation
Modes
Kim, H. Y.; Takizawa, S.; Sasai, H.; Oh, K. Org. Lett. 2017, 19(14),
3867-3870. (DOI)
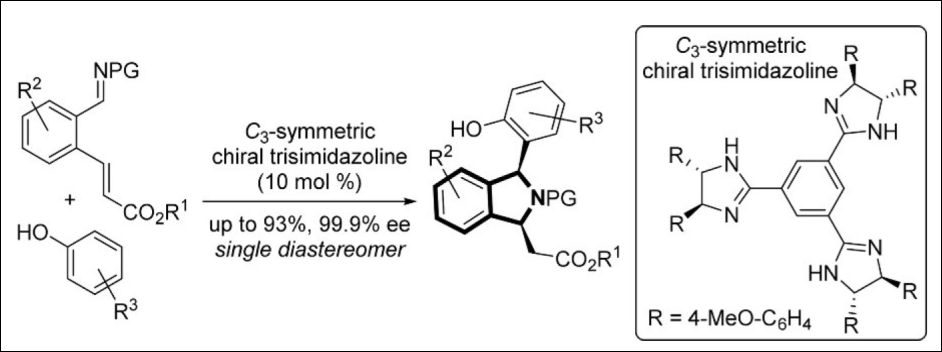
7. Enantio- and
Diastereoselective Betti/aza-Michael Sequence: Single
Operated Preparation of Chiral 1,3-Disubstituted Isoindolines
Takizawa, S.; Sako, M.; Abozeid, M. A.; Kishi, K.; Wathsala, H. D. P.; Hirata,
S.; Murai, K.; Fujioka, H.; Sasai, H. Org. Lett. 2017, 19(19),
5426-5429. (DOI)
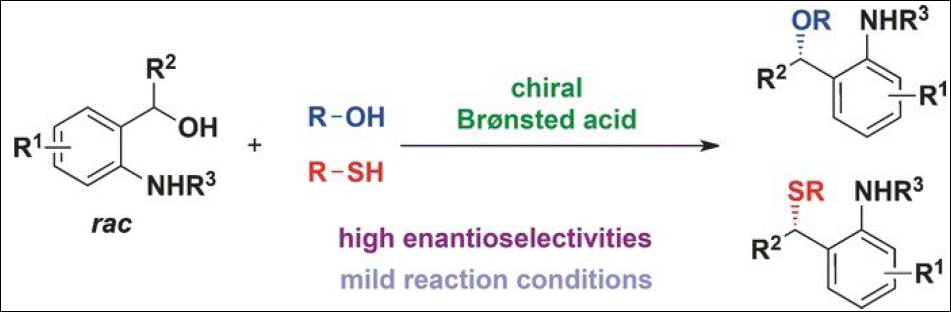
8. Chiral Organocatalyzed
Intermolecular Rauhut–Currier Reaction of Nitroalkenes with Ethyl Allenoate
Takizawa, S.; Sako, M.; Kishi, K.; Shigenobu, M.; Vo-Thanh, G.; Sasai, H. Chem. Pharm. Bull. 2017, 65, 997-999. (DOI)
<Selected as a Cover Picture>
Book &
Review
1. 多機能有機分子触媒を用いるエナンチオ選択的ドミノ反応の開発
滝澤忍、笹井宏明,化学工業,第68巻9号 (2017), 31-38.
2016
Original
Paper
1. Enantioselective
Organocatalytic Oxidation of Ketimines
Takizawa, S.; Kishi, K.; Abozeid, M. A.; Murai, K.; Fujioka, H.; Sasai, H. Org. Biomol. Chem. 2016, 14, 761-767. (DOI)
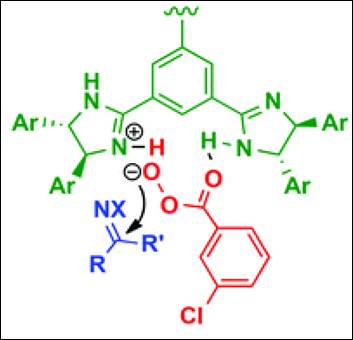
2. Efficient Enantioselective Synthesis of Oxahelicenes Using Redox/Acid Cooperative Catalysts
Sako, M.; Takeuchi, Y.; Tsujihara, T.; Kodera, J.; Kawano, T.; Takizawa, S.;
Sasai, H. J. Am. Chem. Soc. 2016,
138(36), 11481-11484. (DOI)
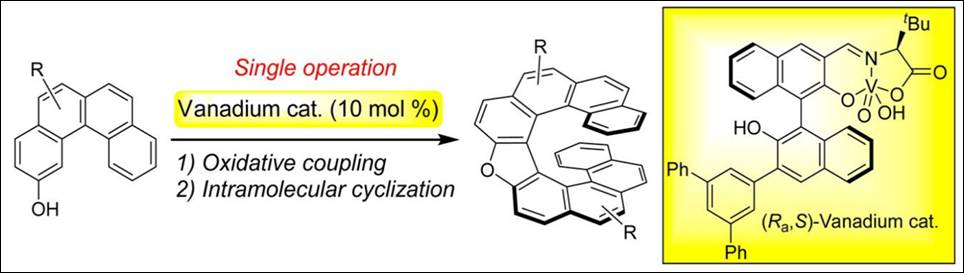
3. Organocatalyzed [4+2] Annulation of
All-Carbon Tetrasubstitued Alkenes with Allenoate:
Synthesis of Highly Functionalized 2H,
and 4H-Pyran Derivatives
Ngo,
T.-Thuy-Duong ; Kishi, K.; Sako, M.; Shigenobu, M.; Bournaud,
C.; Toffano, M.; Guillot, R.; Baltaze,
J.-P.; Takizawa, S.; Sasai, H.; Vo-Thanh, G. ChemistrySelect 2016, 1(17), 5414-5420. (DOI)

Book &
Review
1. “有機分子触媒を用いる脱古典的ドミノ反応の開発動向”
「有機分子触媒の化学 モノづくりのパラダイムシフト」(日本化学会 編)
滝澤忍,化学同人 (2016) 206-207.
Original
Paper
1. Pd-Catalyzed Enantioselective Intramolecularα-Arylation ofα-Substituted Cyclic Ketones: Facile Synthesis of Functionalized Chiral Spirobicycles
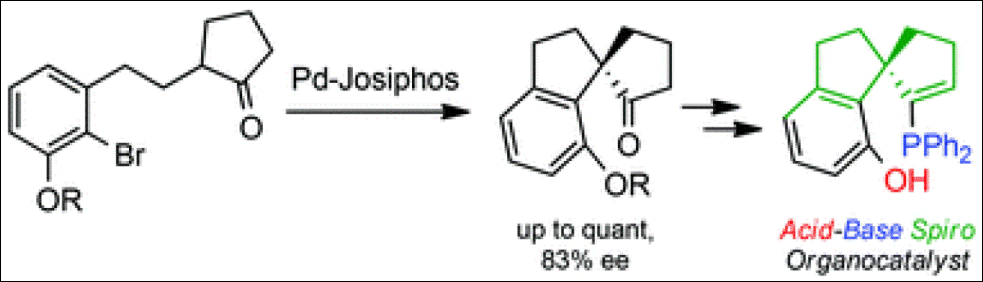
Fan, L.; Takizawa, S.; Takeuchi, Y.; Takenaka, K.; Sasai, H. Org. Biomol. Chem. 2015, 13, 4837-4840. (DOI)
2. Structural Features and Asymmetric Environment of i-Pr-SPRIX Ligand
Takenaka, K.; Lin, X.; Takizawa, S.; Sasai, H. Chirality, 2015,
27, 532-537.. (DOI)

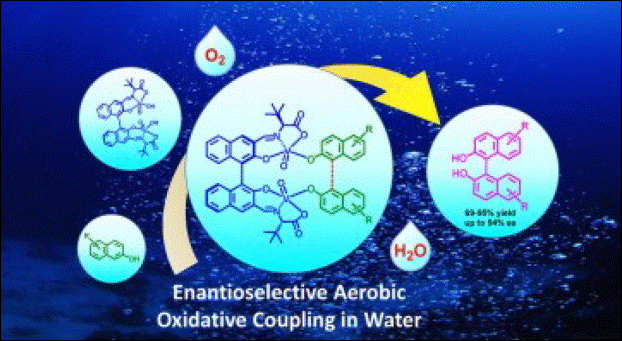
3. Enantioselective and
Aerobic Oxidative Coupling of 2-Naphthol Derivatives Using Chiral Dinuclear Vanadium(V) Complex in Water
Sako, M.; Takizawa, S.; Yoshida, Y.; Sasai, H. Tetrahedron: Asymmetry
2015, 26, 613-616. (DOI)
4. Pd(II)-Catalyzed
Diastereoselective and Enantioselective Domino Cyclization/Cycloaddition
Reactions of Alkenyl Oximes for Polycyclic Heterocycles with Four Chiral Stereogenic Centers
Abozeid, M. A.; Takizawa, S.; Sasai, H. Tetrahedron Lett. 2015, 56, 4316-4319. (DOI)

5. An Enantioselective Organocatalyzed aza-Morita-Baylis-Hillman
Reaction of Isatin-derived Ketimines
with Acrolein
Yoshida, Y.; Sako, M.; Kishi, K.; Sasai, H.; Hatakeyama, S.; Takizawa, S. Org. Biomol. Chem. 2015, 13, 9022-9028. (DOI)

6. Phosphine-Catalyzed β,γ-Umpolung Domino
Reaction of Allenic Esters: Facile Synthesis of
Tetrahydrobenzofuranones Bearing a Chiral Tetrasubstituted Carbon Stereogenic Center
Takizawa, S.;
Kishi, K.; Yoshida, Y.; Mader, S.; Arteaga, F. A.; Lee, S.; Hoshino, M.; Rueping, M.; Fujita, M.; Sasai, H. Angew. Chem. Int. Ed. 2015, 54, 15511-15515. (DOI)
<Highlighted
in Synfacts
2016, 12, 129>
Book &
Review
1. Vanadium in Asymmetric
Synthesis: Emerging Concepts in Catalyst Design and Applications
Takizawa, S.; Gröger, H.; Sasai, H. Chem. Eur.
J. 2015, 21, 8992-8997. (DOI)
2. Sasai, H.; Takizawa, S. “Vanadium
and Niobium-catalyzed Enantioselective Reactions” In Sustainable
Catalysis: With Non-endangered Metals, Part 1, North, M. Ed, Royal
Society of Chemistry; UK, 2015; pp 216-249.
Original
Paper
1. Enantioselective
Oxidative-Coupling of Polycyclic Phenols
Takizawa, S.; Kodera, J.; Yoshida, Y.; Sako, M.; Breukers, S.; Enders, D.; Sasai, H. Tetrahedron 2014, 70, 1786-1793. (DOI)
2. Enantioselective
Organocatalyzed Formal [4+2] Cycloaddition of Ketimines
with Allenoates: Easy Access to a Tetrahydropyridine Framework with a Chiral
Tetrasubstituted Stereogenic Carbon Center
Takizawa, S.; Arteaga, F. A.; Yoshida, Y.; Suzuki, M.; Sasai,
H. Asian J. Org. Chem. 2014, 3, 412-415. (DOI)
3. C3-Symmetric Chiral Trisimidazoline-catalyzed Friedel–Crafts (FC)-type
Reaction
Takizawa, S.; Hirata, S.; Murai, K.; Fujioka, H.; Sasai, H. Org. Biomol. Chem. 2014, 12, 5827-5830. (DOI)
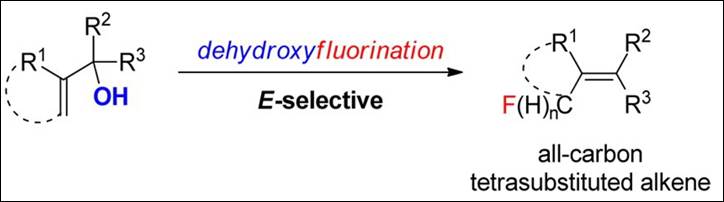
4. Facile Regio- and
Stereoselective Metal-Free Synthesis of All-Carbon Tetrasubstituted Alkenes
Bearing a C(sp3)−F Unit via Dehydroxyfluorination of Morita−Baylis−Hillman (MBH) Adducts
Takizawa, S.; Arteaga, F. A.; Kishi, K.; Hirata,
S.; Sasai, H. Org. Lett. 2014, 16, 4162-4165. (DOI)
Book &
Review
1. Takizawa, S.; Sasai, H. “Metal-catalyzed
Enantio- and Diastereoselective C–C Bond-forming
Reactions in Domino Processes” In Domino Reactions:
Concepts for Efficient Organic Synthesis, Tietze, L. F. Ed, Wiley-VCH Verlag
GmbH & Co. KGaA; Chapter 11, pp. 419-462 (2014).
2. Takizawa, S.; Sasai, H.
"Enantioselective Acid-Base Organocatalyzed Domino Reactions Based on aza-Morita-Baylis-Hillman Process", J. Synth. Org.
Chem. Jpn., vol. 72, No. 7, pp. 781-796
(2014).
Original
Paper
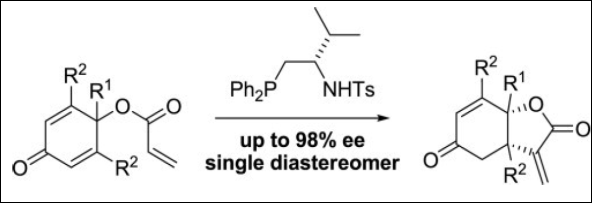
1. Facile Synthesis of α-Methylidene-γ-Butyrolactones: Intramolecular Rauhut-Currier Reaction Promoted by
Chiral Acid-Base Organocatalysts
Takizawa, S.; Nguyen, T. M.-N.; Grossmann, A.; Suzuki, M.; Enders, D.; Sasai H.
Tetrahedron 2013,
69, 1202-1209. (DOI)
2. Vanadium-Catalyzed
Enantioselective Friedel-Crafts-Type Reactions
Takizawa, S.; Arteaga, F. A.; Yoshida, Y.; Kodera, J.;
Nagata, Y.; Sasai, H. Dalton Trans. 2013, 42,
11787-11790. (DOI)
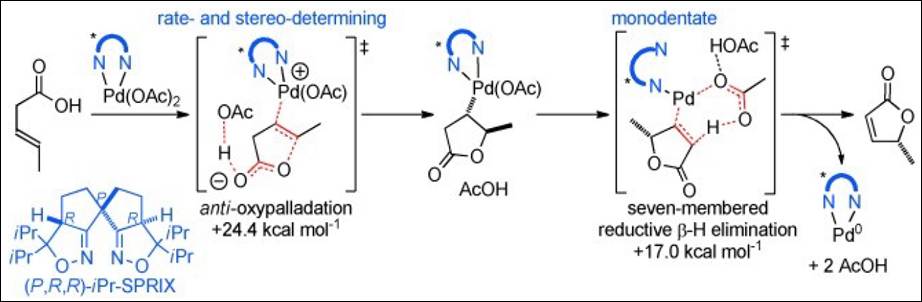
3. DFT
Study on 5-Endo-Trig-Type Cyclization of 3-Alkenoic Acids Using Pd–SPRIX Catalyst: Importance of the Rigid Spiro Framework for Both
Selectivity and Reactivity
Gabr, R. K. M.; Hatakeyama, T.; Takenaka, K.; Takizawa, S.; Okada, Y.;
Nakamura, M.; Sasai, H. Chem. Eur. J. 2013, 19,
9518-9525. (DOI)
4. o-(Hydroxyalkyl)
P-Chirogenic Phosphines as Functional Chiral Lewis
Bases
Rémond, E.; Bayardon,
J.; Takizawa, S.; Rousselin, Y.; Sasai, H.; Jugé,
S. Org. Lett. 2013, 15, 1870-1873. (DOI)
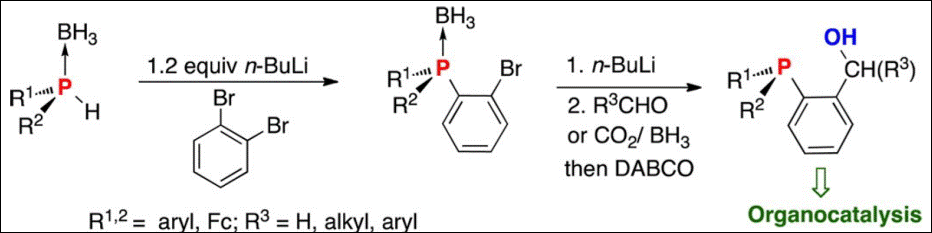
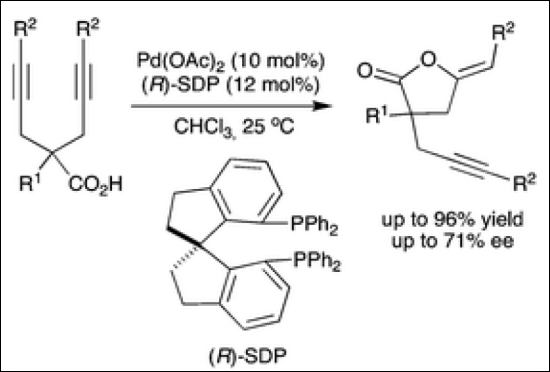
5. Pd(II)-SDP-Catalyzed Enantioselective 5-Exo-Dig Cyclization of γ-Alkynoic Acids: Application to the
Synthesis of Functionalized Dihydrofuran-2(3H)-ones Containing a Chiral
Quaternary Carbon Center
Sridharan,
V.; Fan, L.; Takizawa, S.; Suzuki, T.; Sasai, H. Org. Biomol. Chem. 2013, 11, 5936-5943. (DOI)
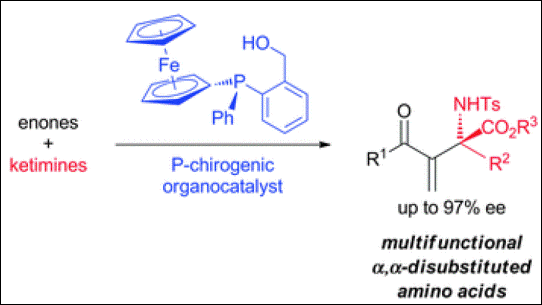
6. P-Chirogenic Organocatalysts:
Application to the aza-Morita-Baylis-Hillman (aza-MBH) Reaction of Ketimines
Takizawa, S.;
Rémond, E.;
Arteaga, F. A.; Yoshida, Y.; Sridharan, V.; Bayardon, J.;
Jugé
S.; Sasai, H. Chem. Commun. 2013, 49, 8392-8394. (DOI)
<Highlighted
in Synfacts
2013, 9, 1297>
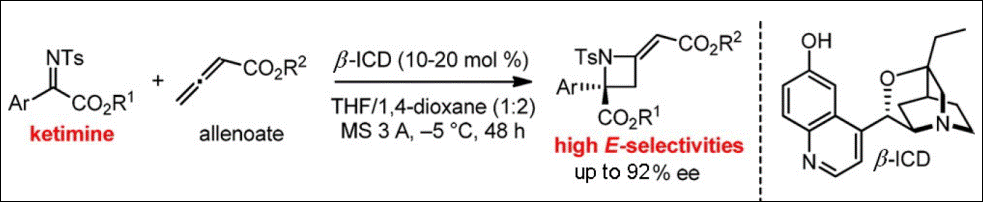
7. Organocatalyzed Formal [2+2] Cycloaddition of Ketimines with Allenoates: Facile Access to Azetidines with
a Chiral Tetrasubstituted Carbon Stereogenic Center
Takizawa, S.; Arteaga, F. A.; Yoshida, Y.; Suzuki, M.; Sasai, H. Org. Lett. 2013,
15, 4142-4145. (DOI)
8. Chiral Bifunctional Organocatalysts
Bearing a 1,3-Propanediamine Unit for the aza-MBH
Reaction
Hirata, S.; Tanaka, K.; Matsui, K.; Arteaga, F. A.; Yoshida, Y.; Takizawa, S.;
Sasai, H. Tetrahedron: Asymmetry 2013,
24, 1189-1192. (DOI)
Original
Paper
1. Enantioselective
Synthesis of a-Alkylidene-g-Butyrolactones:
Intramolecular Rauhut-Currier Reaction Promoted by Acid/Base Organocatalysts
Takizawa, S.; Nguyen, T. M.-N.; Grossmann, A.; Enders, D.; Sasai H. Angew. Chem., Int. Ed. 2012, 51, 5423-5426. (DOI)
<Highlighted
in Synfacts
2012, 8, 787>

2. Design and Synthesis of
Spiro Bis(1,2,3-triazolium) Salts As Chiral Ionic Liquids
Yoshida, Y.; Takizawa, S.; Sasai, H. Tetrahedron: Asymmetry 2012, 23, 843-851. (DOI)

Book &
Review
1. Sasai, H. and Takizawa, S. “C-C Bond Formation: (aza)
Morita-Baylis-Hillman Reaction” in Comprehensive
Chirality, Carreira, E. M. and Yamamoto, H. Ed., Elsevier B. V.; Amsterdam, 6,
pp. 234-263 (2012).
Original
Paper
1. An
Enantioselective Organocatalyzed aza-MBH Domino
Process: Application to the Facile Synthesis of Tetrahydropyridines
Takizawa, S.; Inoue, N.; Sasai H. Tetrahedron Lett. 2011, 52, 377-380. (DOI)
2. Chlorinative Cyclization of 1,6-Enynes
by Enantioselective Palladium(II)/Palladium(IV) Catalysis
Takenaka, K.; Hashimoto, S.; Takizawa, S.; Sasai, H. Adv. Synth. Catal. 2011, 353,
1067-1070. (DOI).
3. Enantioselective
Cyclization of 4-Alkenoic Acids via an Oxidative Allylic C-H Esterification
Takenaka, K.; Akita, M.; Tanigaki, Y.; Takizawa, S.; Sasai, H. Org. Lett. 2011, 13,
3506-3509. (DOI).
<Highlighted
in Synfacts 2011, 991>
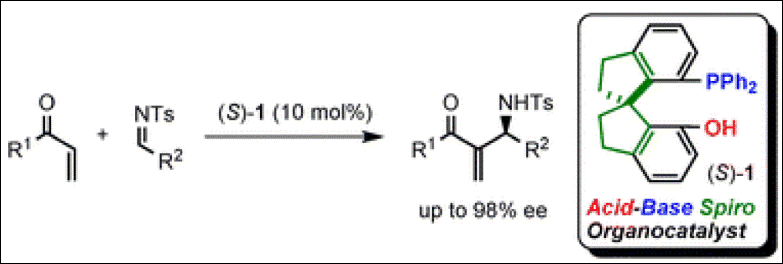
4. A Bifunctional Spiro-Type Organocatalyst with High Enantiocontrol: Application to the
Aza-Morita-Baylis-Hillman Reactions
Takizawa, S.; Kiriyama, K.; Ieki, K.; Sasai, H. Chem. Commun. 2011, 47,
9227-9229. (DOI).
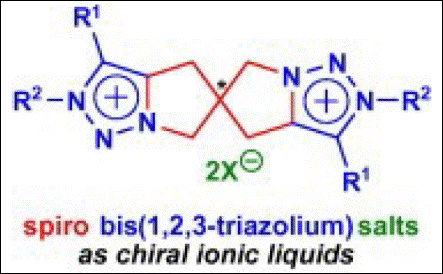
5. Synthesis of Spiro
Bis(1,2,3-triazolium) Salts As Chiral Ionic Liquids
Yoshida, Y.; Takizawa, S.; Sasai, H. Tetrahedron Lett. 2011, 52, 6877-6879. (DOI).
Book &
Review
1.
Immobilization of Multicomponent Asymmetric
Catalysts (MACs)
「Polymeric Chiral Catalyst Design and Chiral Polymer Synthesis」(Itsuno, S. Ed.)
Takizawa, S.; Sasai, H. John Wiley & Sons, (2011) 293-322.
Original
Paper
1. Asymmetric Synthesis of
Chiral Spiro Bis(isoxazoline) and Spiro (Isoxazole-Isoxazoline) Ligands
Takenaka, K.; Nagano, T.; Takizawa, S.; Sasai, H. Tetrahedron:
Asymmetry 2010, 21, 379-381. (DOI)
2. Enantioselective
6-Endo-Trig Wacker-Type Cyclization of 2-Geranylphenols: Application to Facile
Synthesis of (−)-Cordiachromene
Takenaka, K.; Tanigaki, Y.; Patil, M. L.; Rao, C. V. L.; Takizawa, S.; Suzuki, T.; Sasai, H. Tetrahedron: Asymmetry 2010, 21, 767-770. (DOI)
3. Acid-Base Organocatalysts for the Aza-Morita-Baylis-Hillman Reaction
of Nitroalkenes
Takizawa, S.; Horii, A.; Sasai, H. Tetrahedron: Asymmetry 2010, 21, 891-894.
(DOI)
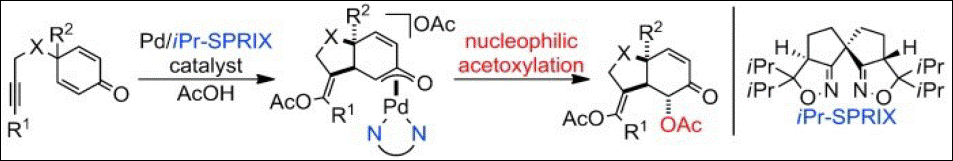
4. Enantioselective
Wacker-Type Cyclization of 2-Alkenyl-1,3-Diketones Promoted by Pd-SPRIX
Catalyst
Takenaka, K.; Mohanta, S. C.; Patil, M. L.; Rao, C. V. L.; Takizawa, S.;
Suzuki, T.; Sasai, H. Org. Lett. 2010, 12, 3480-3483. (DOI)
<Highlighted
in Synfacts 2010, 1263>
5. Enantioselective
Synthesis of Isoindolines: Organocatalyzed Domino
Process Based on the aza-Morita-Baylis-Hillman (aza-MBH) Reaction
Takizawa, S.; Inoue, N.; Hirata, S.; Sasai H. Angew. Chem. Int. Ed. 2010, 49, 9725-9729. (DOI)
<Highlighted
in Synfactcs 2011, 221>
6. Pd-Catalyzed
5-Endo-Trig-Type Cyclization of b,g-Unsaturated Carbonyl
Compounds: an Efficient Ring Closing Reaction to Give g-Butenolides and
3-Pyrrolin-2-ones
Bajracharya, G. B.; Koranne, P. S.; Nadaf, R. N.; Gabr, R. K. M.; Takenaka, K.;
Takizawa, S.; Sasai H. Chem. Commun. 2010, 46, 9064-9066. (DOI)
Book &
Review
1.
「使える!有機合成反応241実践ガイド」(丸岡啓二, 野崎京子, 石井康敬, 大寺純蔵, 富岡清 編著)
笹井宏明, 滝澤忍, 竹中和浩, 化学同人 (2010) 224-225, 262-263, 280-281,
360-361.
Original
Paper
1.
Dicationic Palladium(II)-Spiro bis(isoxazoline) Complex
for Highly Enantioselective Isotactic Copolymerization of CO with Styrene
Derivatives
Bajracharya, G. B.; Koranne, P. S.; Tsujihara, T.; Takizawa, S.;
Onitsuka, K.; Sasai, H. Synlett 2009, 310-314. (DOI)
2. One-pot Preparation of
Chiral Dinuclear Vanadium(V) Complex
Takizawa, S.; Rajesh, D.; Katayama, T.; Sasai, H. Synlett 2009,
1667-1669. (DOI)
3. Enantioselective
Intramolecular Oxidative Aminocarbonylation of Alkenylureas Catalyzed by Palladium-Spiro Bis(isoxazoline) Complexes
Tsujihara, T.; Shinohara, T.; Takenaka, K.; Takizawa, S.; Onitsuka, K.;
Hatanaka, M.; Sasai, H. J. Org. Chem. 2009, 74,
9274-9279. (DOI)
Book &
Review
1.
“第10章 酸塩基複合型触媒 アザMBH反応を中心として”
「化学フロンティア21 進化を続ける有機触媒−有機合成を革新する第三の触媒」 (丸岡啓二 編)
笹井宏明, 滝澤忍, 化学同人 (2009) 120-127.
2.
複数の構成要素を持つ不斉触媒“Multicomponent Asymmetric Catalyst (MAC)”の固定化
滝澤忍, 荒井孝義, 笹井宏明, 有機合成化学協会誌, 第67巻3号 (2009), 194-207.
3.
Development of Dinuclear Vanadium
Catalysts for Enantioselective Coupling of 2-Naphthols via a Dual Activation
Mechanism
Takizawa, S. Chem. Pharm. Bull. 2009, 57,
1179-1188.
4.
二重活性化能を有する酸塩基型不斉有機分子触媒の開発とaza-Morita-Baylis-Hillman反応への展開
滝澤忍, 薬学雑誌, 第129巻10号 (2009), 1201-1210.
5.
Development of Chiral Spiro Ligands for
Metal-Catalyzed Asymmetric Reactions
Bajracharya, G. B.; Arai, M. A.; Koranne, P. S.; Suzuki, T.; Takizawa, S.;
Sasai, H. Bull.
Chem. Soc. Jpn. 2009, 82, 285-302. (DOI)
Original Paper
1.
Dual Activation in Oxidative Coupling of
2-Naphthols Catalyzed by Chiral Dinuclear Vanadium
Complexes
Takizawa, S.; Katayama, T.; Somei, H.; Asano, Y.;
Yoshida, T.; Kameyama, C.; Rajesh, D.; Onitsuka, K.; Suzuki, T.; Mikami, M.; Yamataka, H.; Jayaprakash, D.; Sasai, H. Tetrahedron 2008,
64, 3361-3371. (DOI)
2.
Chiral Dinuclear
Vanadium(V) Catalysts for Oxidative Coupling of 2-Naphthols
Takizawa, S.; Katayama, T.; Kameyama, C.; Onitsuka, K.; Suzuki, T.; Yanagida,
T.; Kawai, T.; Sasai, H. Chem. Commun. 2008, 1810-1812. (DOI)
<Highlighted
in Synfacts 2008, 737>
3.
Divergent Synthesis of Chiral Spiro
(Isoxazole-Isoxazoline) Hybrid Ligands
Takenaka, K.; Nakatsuka, S.; Tsujihara, T.; Koranne, P. S.; Sasai, H. Tetrahedron: Asymmetry 2008,
19, 2492-2496. (DOI)
Book &
Review
1.
Recent Development on Chiral Ionic Liquids: Design,
Synthesis, and Applications
Patil, M. L.; Sasai, H. Chem. Rec. 2008, 8, 98-108. (DOI)
2.
Dinuclear Chiral
Vanadium Catalysts for Oxidative Coupling of 2-Naphthols via a Dual Activation
Mechanism
Takizawa, S.; Katayama, T.; Sasai, H. Chem. Commun. 2008,
4113-4122. (DOI)
Original Paper
1.
Synthesis
of Novel Spiro Imidazolium Salts as Chiral Ionic Liquids
Patil, M. L.; Rao, C. V. L.; Takizawa, S.; Takenaka, K.; Onitsuka, K.; Sasai,
H. Tetrahedron 2007,
63, 12702-12711. (DOI)
2.
Development
of New Methods towards Efficient Immobilization of Enantioselective Catalysts
Takizawa, S.; Patil, M. L.; Marubayashi, K.; Sasai,
H. Tetrahedron 2007,
63, 6512-6528. (DOI)
3.
Enantioselective Glyoxylate-ene Reaction using a Novel Spiro Bis(isoxazoline)
Ligand in Copper Catalysis
Wakita, K.; Bajracharya, G. B.; Arai, M. A.; Takizawa, S.; Suzuki, T.; Sasai,
H. Tetrahedron: Asymmetry 2007, 18, 372-376. (DOI)
4.
Optical Resolution of Tetra
Isopropyl-substituted Spiro Bis(isoxazoline) i-Pr-SPRIX
Takizawa, S.; Yogo, J.; Tsujihara, T.; Onitsuka, K.; Sasai, H. J. Organomet. Chem. 2007, 692, 495-498. (DOI)
Book &
Review
1.
Bifunctional Organocatalysts
for Enantioselective Aza-Morita-Baylis-Hillman (Aza-MBH) Reactions
Takizawa, S.; Matsui, K.; Sasai, H. J. Synth. Org. Chem. Jpn. 2007, 65 (11),
1089-1098.
2.
酸−塩基型不斉有機分子触媒を用いるaza-Morita-Baylis-Hillman反応
笹井宏明, 滝澤忍, 松井嘉津也, THE CHEMICAL TIMES KANTO CHEMICAL CO.
INC. 2007, 1, 3-8.
Original Paper
1.
Design and Synthesis of Novel Chiral Spiro
Ionic Liquids
Patil, M. L.; Rao, C. V. L.; Yonezawa, K.; Takizawa, S.; Onitsuka, K.; Sasai,
H. Org. Lett. 2006, 8, 227-230. (DOI)
2.
Conformational Lock in Brfnsted Acid - Lewis Base Organocatalyst for the aza-Morita-Baylis-Hillman
Reaction
Matsui, K.; Tanaka, K.; Horii, A.; Takizawa, S.; Sasai, H. Tetrahedron: Asymmetry 2006,
17, 578-583. (DOI)
3.
A Brfnsted Acid - Lewis Base Organocatalyst for the aza-Morita-Baylis-Hillman
Reaction
Matsui, K.; Takizawa, S.; Sasai, H. Synlett 2006,
761-765. (DOI)
Book &
Review
1.
“第18章 酸−塩基型不斉有機分子触媒によるaza-Morita-Baylis-Hillman反応”
「有機分子触媒の新展開」(柴崎正勝 監修)
笹井宏明, 滝澤忍, 松井嘉津也, シ−・エム・シー出版 (2006) 231-242.
2.
Development of Efficient Methods for the Immobilisation of Multicomponent Asymmetric Catalysts
Jayaprakash, D.; Takizawa, S.; Arai, T.; Sasai, H. J. Experimental
Nanoscience 2006, 1, 477-510. (DOI)
Patent
1.
「光学活性スピロビスイソオキサゾリン誘導体とその製造方法およびその金属錯体を用いた不斉触媒反応」
笹井宏明, 脇田和彦, 加藤孝浩, 荒井緑, 特開2006-76939
2.
「光学活性スピロビスイソオキサゾール誘導体およびその製造法、並びにその金属錯体を用いた不斉触媒反応」
笹井宏明, 脇田和彦, 加藤孝浩, 荒井緑, 特開2006-76915
3.
「スピロキラリティを有する第4級アンモニウム塩およびその製造法、並びに該アンモニウム塩を用いた不斉触媒反応」
下元愛, 米澤浩司, 滝澤忍, 笹井宏明, 特開2006-76911
4.
「新規スピロ構造化合物とその製造法」
マヘッシュ エル パティル, シラムコッティ ベンカット ラクシュマン ラオ, 滝澤忍, 笹井宏明, 特開2006-76887
5.
「スピロ骨格を持つキラルな相間移動触媒およびその製造法、並びにそれを用いた不斉触媒反応」
米澤浩司, 下元愛, 滝澤忍, 笹井宏明, 特開2006-70001
6.
「Novel organic molecular catalyst having
binaphthol skeleton and processes for producing the same and application
thereof」
Sasai Hiroaki, Takizawa Shinobu, Matsui Katsuya, Patent No. US 2006-009646
7.
「ビナフトール骨格を有する新規有機分子触媒およびその製造法と応用」
笹井宏明, 滝澤忍, 松井嘉津也, 特開2006-28021
Original Paper
1.
Spiro Crown Ethers Bearing (S )-1,1'-Spirobiindanes
as Chiral Backbones
Yonezawa, K.; Patil, M. L.; Sasai, H.; Takizawa, S. Heterocycles 2005, 66, 639-644.
2.
Bifunctional Organocatalysts
for Enantioselective aza-Morita-Baylis-Hillman
Reaction
Matsui, K.; Takizawa, S.; Sasai, H. J. Am. Chem. Soc. 2005, 127, 3680-3681. (DOI)
3.
Micelle-Derived Polymer Supports for
Enantioselective Catalysts
Takizawa, S.; Patil, M. L.; Yonezawa, F.; Marubayashi,
K.; Tanaka, H.; Kawai, T.; Sasai, H. Tetrahedron Lett. 2005, 46, 1193-1197. (DOI)
4.
Enantioselective Morita-Baylis-Hillman (MBH)
Reaction Promoted by a Heterobimetallic Complex with a Lewis Base
Matsui, K.; Takizawa, S.; Sasai, H. Tetrahedron Lett. 2005, 46, 1943-1946. (DOI)
Original Paper
1.
Dual Activation in a Homolytic Coupling
Reaction Promoted by an Enantioselective Dinuclear
Vanadium(IV) Catalyst
Somei, H.; Asano, Y.; Yoshida, T.; Takizawa, S.; Yamataka, H.; Sasai, H. Tetrahedron Lett. 2004, 45, 1841-1844. (DOI)
2.
Development of Novel Chiral Spiro Ligand
Bearing Oxazolines
Kato, T.; Marubayashi, K.; Takizawa, S.; Sasai, H. Tetrahedron: Asymmetry 2004,
15, 3693-3697. (DOI)
3.
Enantioselective Aldol-type Reaction Using
Diketene
Kawase, T.; Takizawa, S.; Jayaprakash, D.; Sasai, H. Synth. Commun. 2004,
34, 4487-4492. (DOI)
Book &
Review
1.
Asymmetric ligands bearing spiro skeleton and
their applications to enantioselective catalysis
Takizawa, S.; Jayaprakash, D.; Patil, M. L.; Muthiah, C.; Sasai, H. Materials Integration 2004,
17, 3-6.
2.
Development of novel immobilization methods
for multifunctionl asymmetric catalysts
Takizawa, S.; Sasai, H. 生産と技術 2004, 56, 43.
3.
Trend in the development of novel chiral
ionic liquids
Patil, M. L.; Takizawa, S.; Sasai, H. Chemical Industry 2004, 55, 877.
Original Paper
1.
Design and Synthesis of Novel Spiro
Pyridinium and Quinolinium Salts
Patil, M. L.; Takizawa, S.; Sasai, H. Heterocycles 2003, 61, 581.
2.
Metal-bridged Polymers as Insoluble
Multicomponent Asymmetric Catalysts with High Enantiocontrol: An Approach for
the Immobilization of Catalysts without Using any Support
Takizawa, S.; Somei, H.; Jayaprakash, D.; Sasai, H. Angew. Chem. Int. Ed. 2003, 42, 5711. (DOI)
3.
Monolayer-protected Au Cluster
(MPC)-supported Ti-BINOLate Complex
Marubayashi, K.; Takizawa, S.; Kawakusu,
T.; Arai, T.; Sasai, H. Org. Lett. 2003, 5, 4409. (DOI)
4.
Synthesis of Novel Chiral Spiro Bis(pyrazole)
Ligands
Takizawa, S.; Honda, Y.; Arai, M. A.; Kato, T.; Sasai, H. Heterocycles 2003,
60, 2551.
5.
Polymer Supported BisBINOL
Ligands for the Immobilization of Multicomponent Asymmetric Catalysts
Sekiguti, T.; Iizuka, Y.; Takizawa, S.; Jayaprakash,
D.; Arai, T.; Sasai, H. Org. Lett. 2003, 5, 2647. (DOI)
6.
Enantioselective Synthesis of a-Methylene-g-butyrolactones Using
Chiral Pd(II)-SPRIX Catalyst
Muthiah, C.; Arai, M. A.; Shinohara, T.; Arai, T.; Takizawa, S.; Sasai, H. Tetrahedron Lett. 2003,
44, 5201. (DOI)
7.
"Catalyst Analogue": A Concept for
Constructing Multicomponent Asymmetric Catalysts (MAC) Using a Polymer Support
Arai, T.; Sekiguti, T.; Otsuki, K.; Takizawa, S.;
Sasai, H. Angew. Chem. Int. Ed. 2003,
42, 2144. (DOI)