We are engaged in the creation of organic compounds that express intended medicinal effects (drug discovery) based on organic chemistry. We are engaged in drug discovery chemistry, medicinal chemistry, and chemical biology research, utilizing our broad knowledge of synthetic organic chemistry, computational chemistry, structural biology, biochemistry, molecular biology, and genetics to synthesize new biologically active compounds and elucidate their mechanisms of action.
interview :The challenge of making medicine (JP)
1.Drug discovery research of epigenetics-regulated compounds
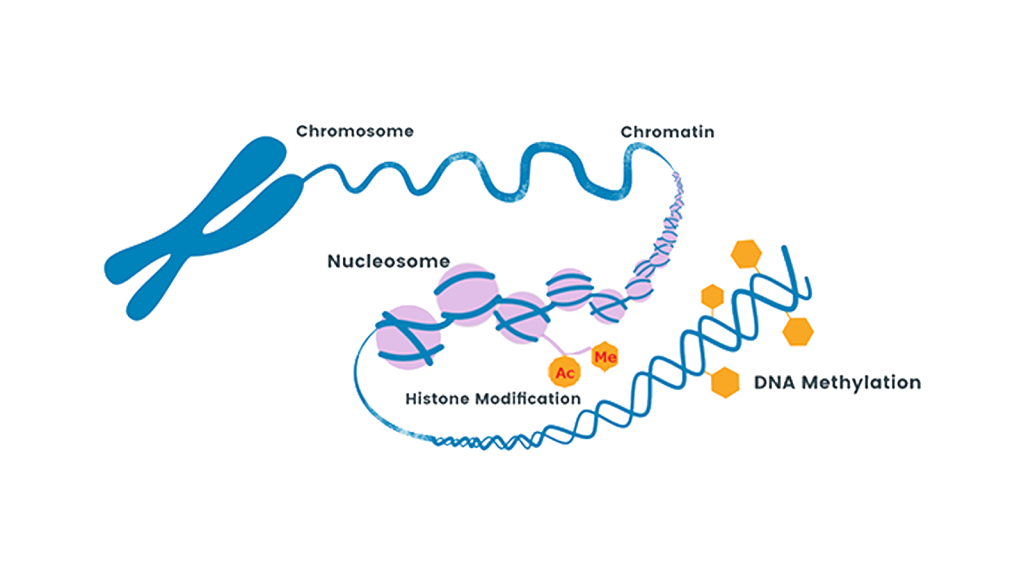
Mechanisms that regulate gene expression independently of the DNA sequence are called epigenetics. Recent studies have revealed that methylation of cytosine and acetylation and methylation of histone lysine residues are important epigenetic mechanisms. Epigenetic abnormalities have also been reported to be involved in diseases such as cancer. DNA methyltransferase inhibitors and histone deacetylase (HDAC) inhibitors have been developed and clinically applied. Our research group is conducting research on the creation of isozyme-selective HDAC inhibitors and histone demethyltransferase inhibitors with the aim of developing next-generation epigenetic drugs.
Epigenetics-regulating compounds we have developed so far
HDAC inhibitor NCH-51
HDAC8 selective inhibitor NCC-149
HDAC3 selective inhibitor T247
LSD1 inhibitor NCL-1
KDM inhibitor NCDM-32b
KDM2/7 inhibitor NCDM-64
※Epigenetics control compounds developed so far in the Suzuki lab can be purchased from reagent companies.
2.Drug Discovery Chemistry Research with Click Chemistry
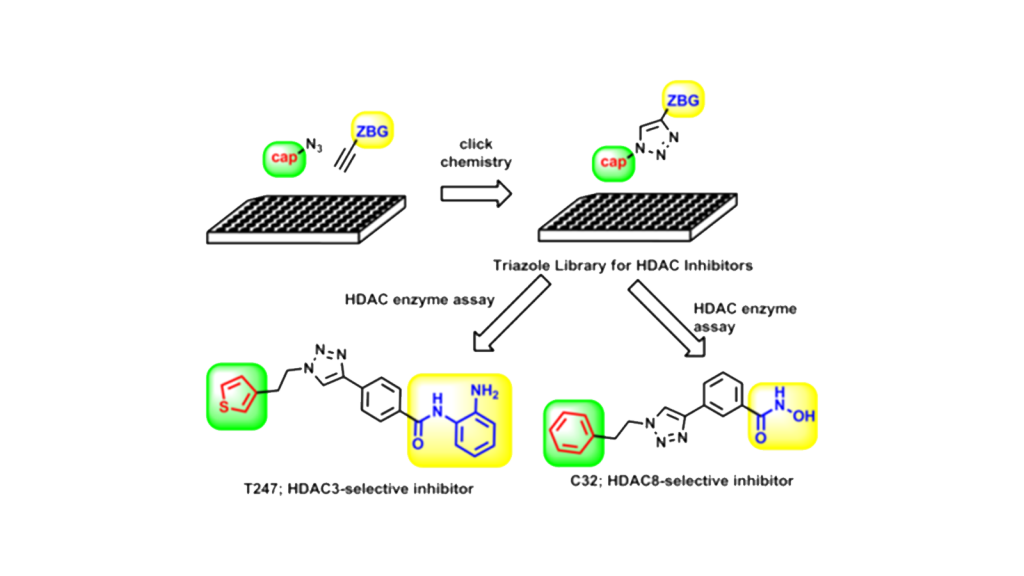
Click chemistry is a technique for creating new functional molecules by utilizing reactions that combine multiple compounds in an efficient and simple manner. In our laboratory, we are using click chemistry to construct a library of low-molecular-weight compounds and conduct exploratory research for new bioactive substances. We have constructed a library for histone deacetylase (HDAC) inhibitors using copper-catalyzed azide-alkyne cycloaddition (CuAAC), a typical reaction in click chemistry, and have developed HDAC isozyme-specific inhibitors (HDAC3 and HDAC8-specific inhibitors).
3.Target enzyme-directed inhibitor synthesis
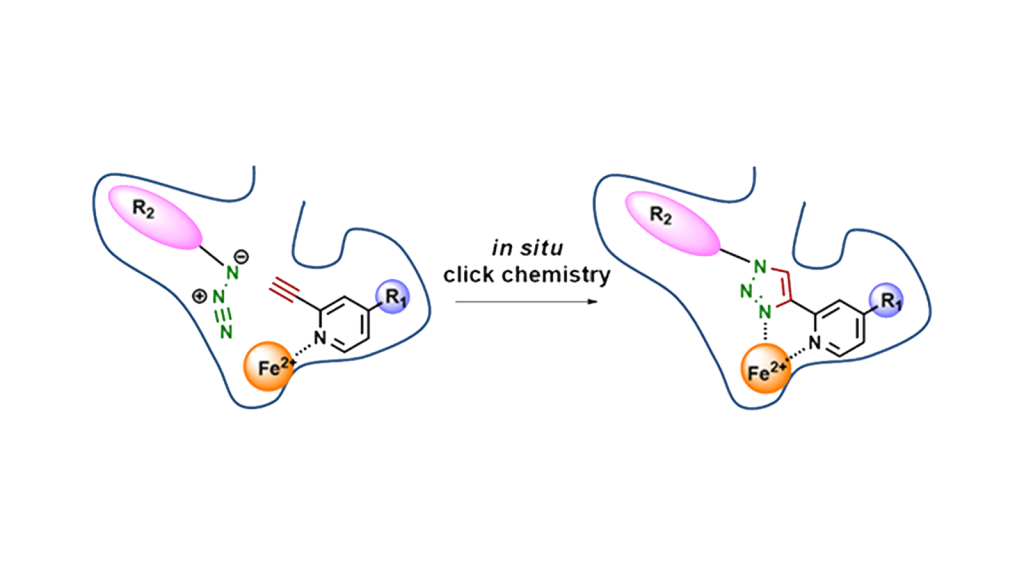
We are attempting to create enzyme-specific inhibitors using targeted enzyme-guided synthesis (TGS), in which an inhibitor is synthesized in situ by the enzyme itself using a catalytic reaction triggered by the target enzyme or a pocket specific to that enzyme. TGS in this study is more likely to produce inhibitors that are specific to the enzyme because the inhibitor is produced within the enzyme. So far, we have succeeded in creating inhibitors of SIRT1 and HDAC8, histone deacetylases, and LSD1, histone demethylase, by TGS.
4.Analytical study of non-covalent interactions for drug discovery applications
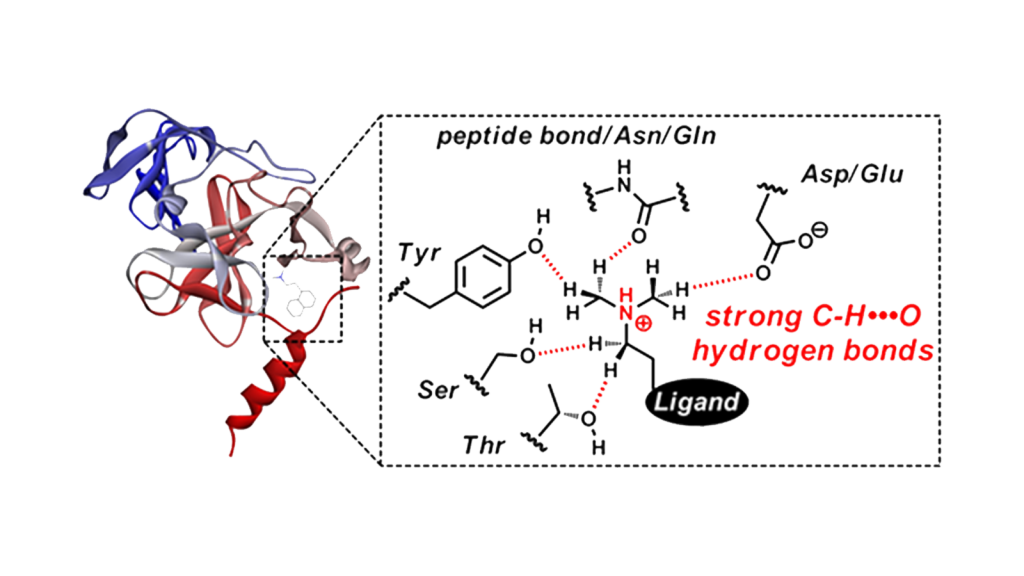
Noncovalent interactions of hydrogen bonds and hydrophobic interactions play an important role in reversible binding between ligands and target proteins. In our laboratory, ligand-protein interaction analysis is performed by analytical chemistry methods using computational science and small molecule models.
